Animal Health Safety
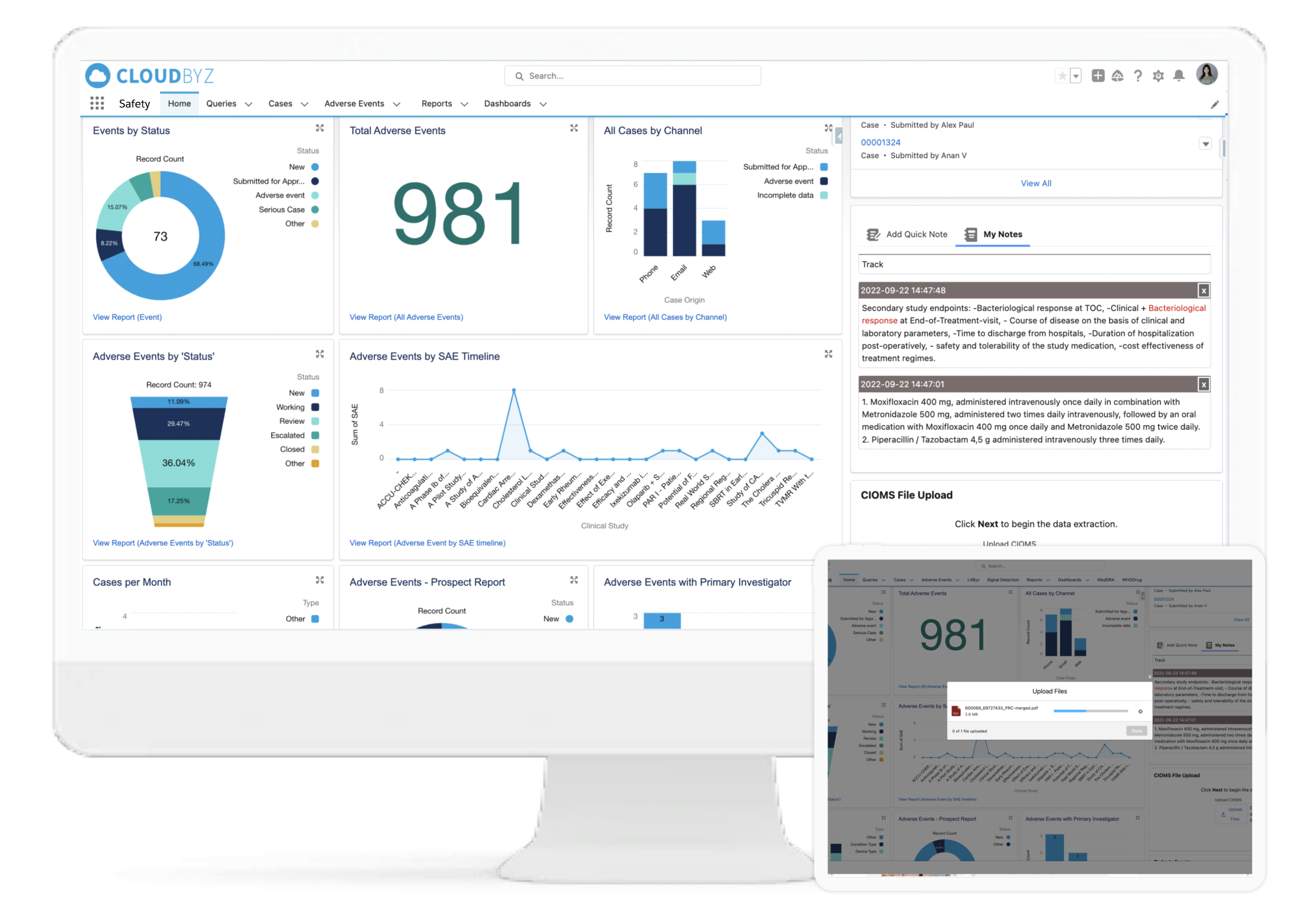
The Cloudbyz Animal Health Safety platform is a cloud-native pharmacovigilance and safety solution purpose-built to support pre- and post-approval safety tracking in animal health clinical trials. From managing VeDDRA-coded adverse event reports to maintaining GCP compliant audit trails, the platform supports the complete lifecycle of safety data across species, studies, and global markets.
Designed for use by sponsors and CROs involved in Target Animal Safety Studies (TASS), TAES, and post-market surveillance, Cloudbyz helps ensure regulatory readiness while improving visibility and operational efficiency.
Product Features
Species-Specific AE/SAE Tracking
Track adverse events and serious adverse events using built-in VeDDRA-coded dictionaries to support species-specific accuracy. Custom fields and structured templates allow recording of organ function, behavioral indicators, and other animal health safety metrics across species and breeds.
Species-Specific AE/SAE Tracking
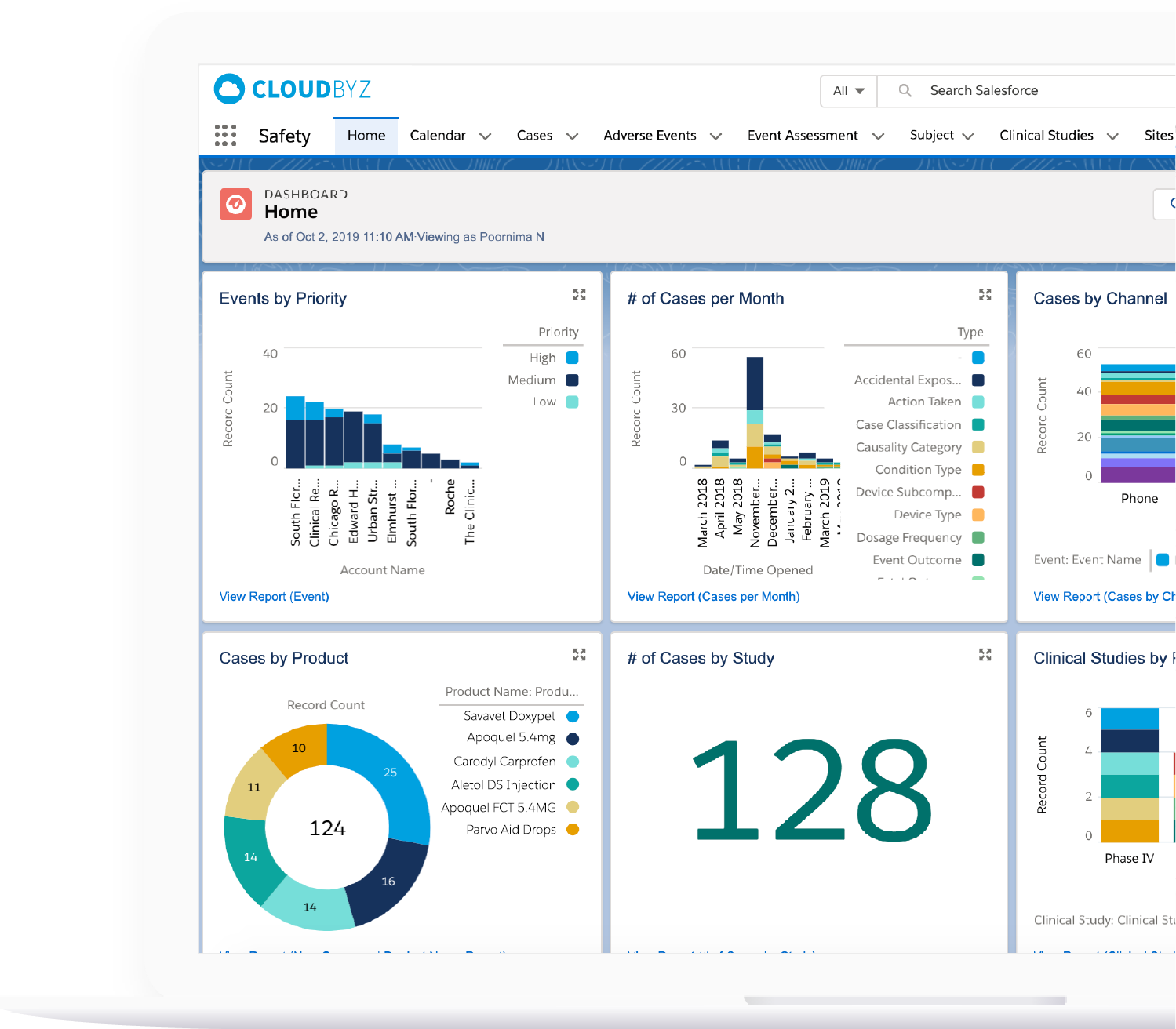
Unified Safety Case Management

Global Reporting & Submission Readiness
Owner-Reported Outcomes & Vet Input Forms
Enable easy collection of owner feedback on suspected adverse events via structured digital forms. Veterinary staff can validate and annotate owner entries, ensuring structured and validated input in real time.
Owner-Reported Outcomes & Vet Input Forms
Key Benefits
Built-in compliance with CVM, CVMP, and GCP standards
VeDDRA-coded terminology for cross-species accuracy
Configurable alerts and validation rules for proactive monitoring
Unified platform with seamless integration into Cloudbyz CTMS, EDC, and RTSM modules
Easy collaboration between CROs, sponsors, veterinarians, and regulators

.png)