Clinical Trial Financial Management
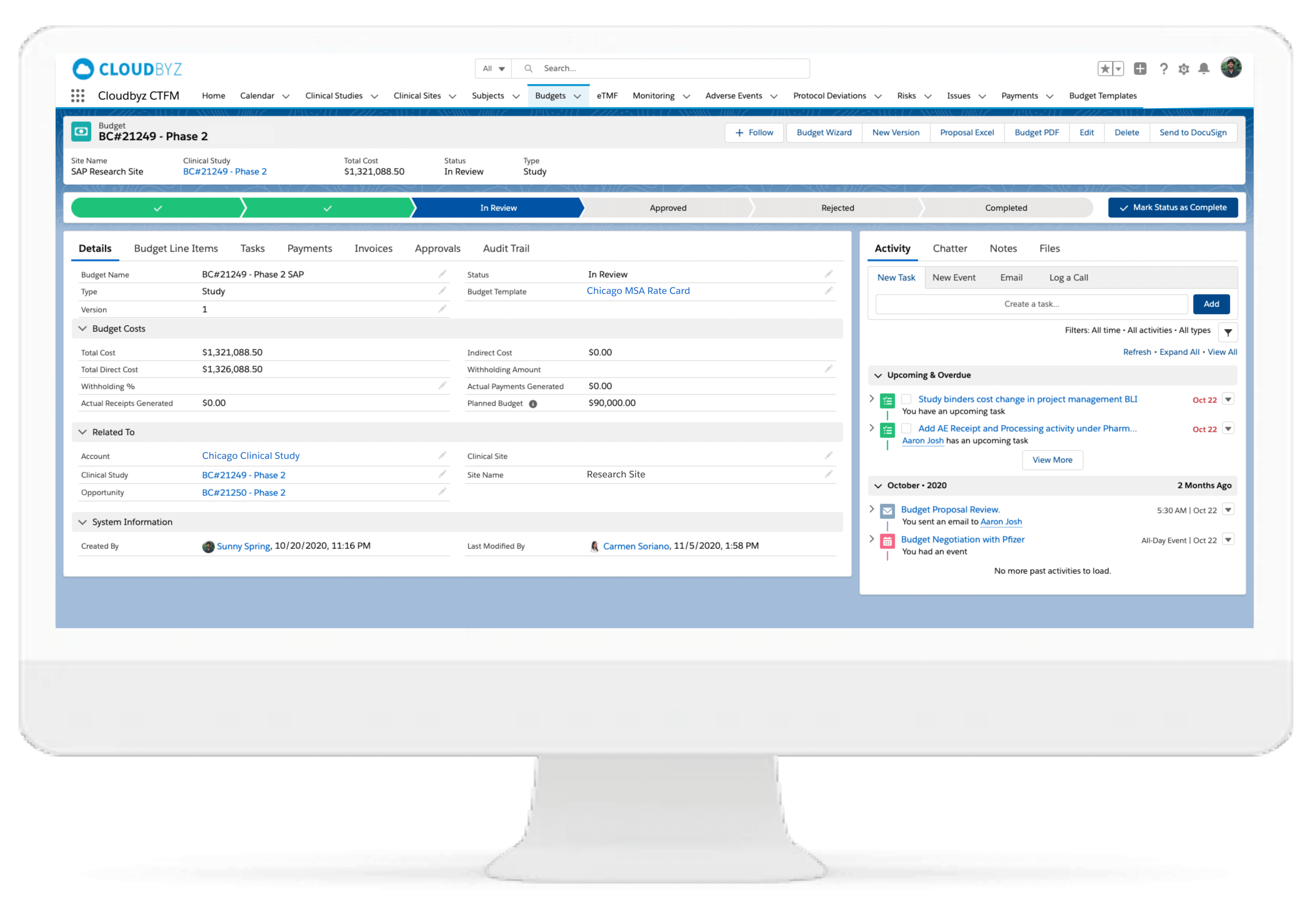
Animal health clinical studies involve complex budgeting requirements—from multi-species dosing logistics to investigator reimbursements and field-based operational costs. Our Clinical Trial Financial Management (CTFM) module is specifically designed for animal health research, offering sponsors and CROs real-time visibility and control over trial costs, investigator payments, and vendor allocations across sites and geographies.
Product Features
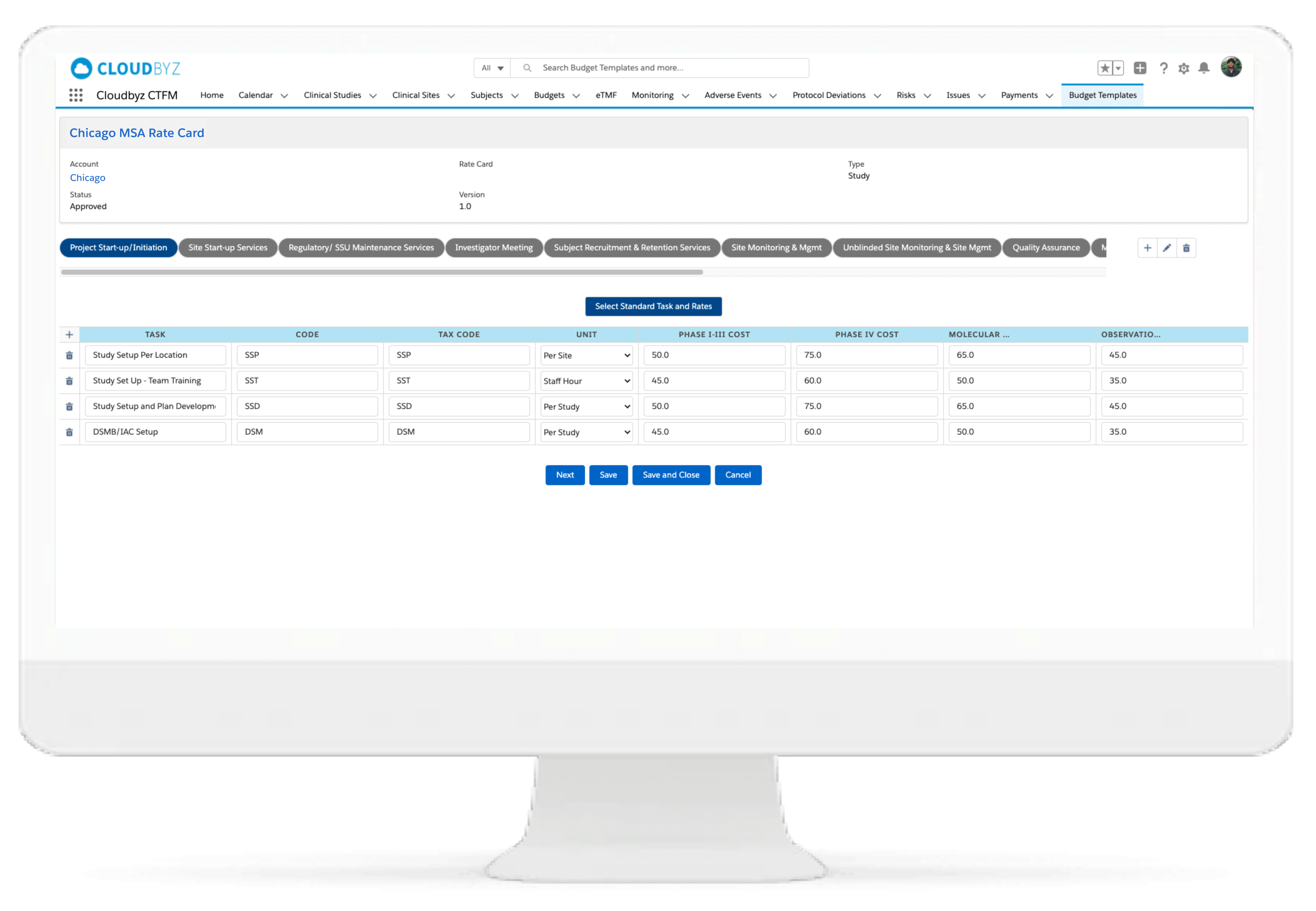
Multi-Species Budgeting Framework
Create detailed budgets by species, breed, and treatment arm. Account for study-specific parameters like customized dosing schedules (e.g., 1x, 3x, 5x) and additional site monitoring for high-risk breeds or geographies.
Multi-Species Budgeting Framework
Why Cloudbyz CTFM for Animal Health Trials?
Built for Animal Health Studies
Designed around species-specific workflows, TAES/TASS protocols, and real-world animal health site operations.
Supports Global Compliance
Aligns with CVM, CVB, and EMA financial documentation requirements, including transparency for cost reimbursement.
Field-Tested Flexibility
Supports site, field, and lab budgets—whether your trial involves household pets or livestock in rural environments.
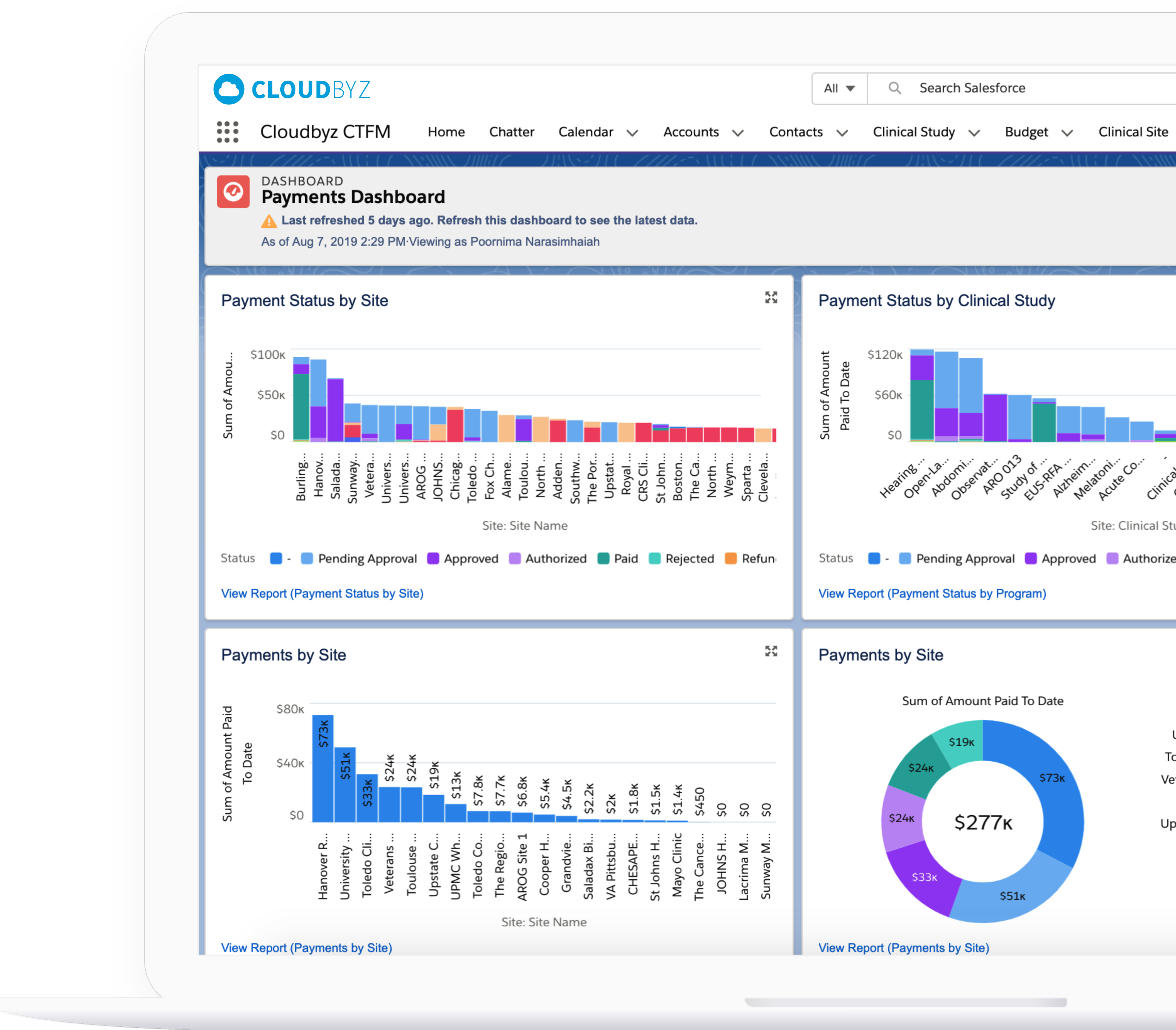
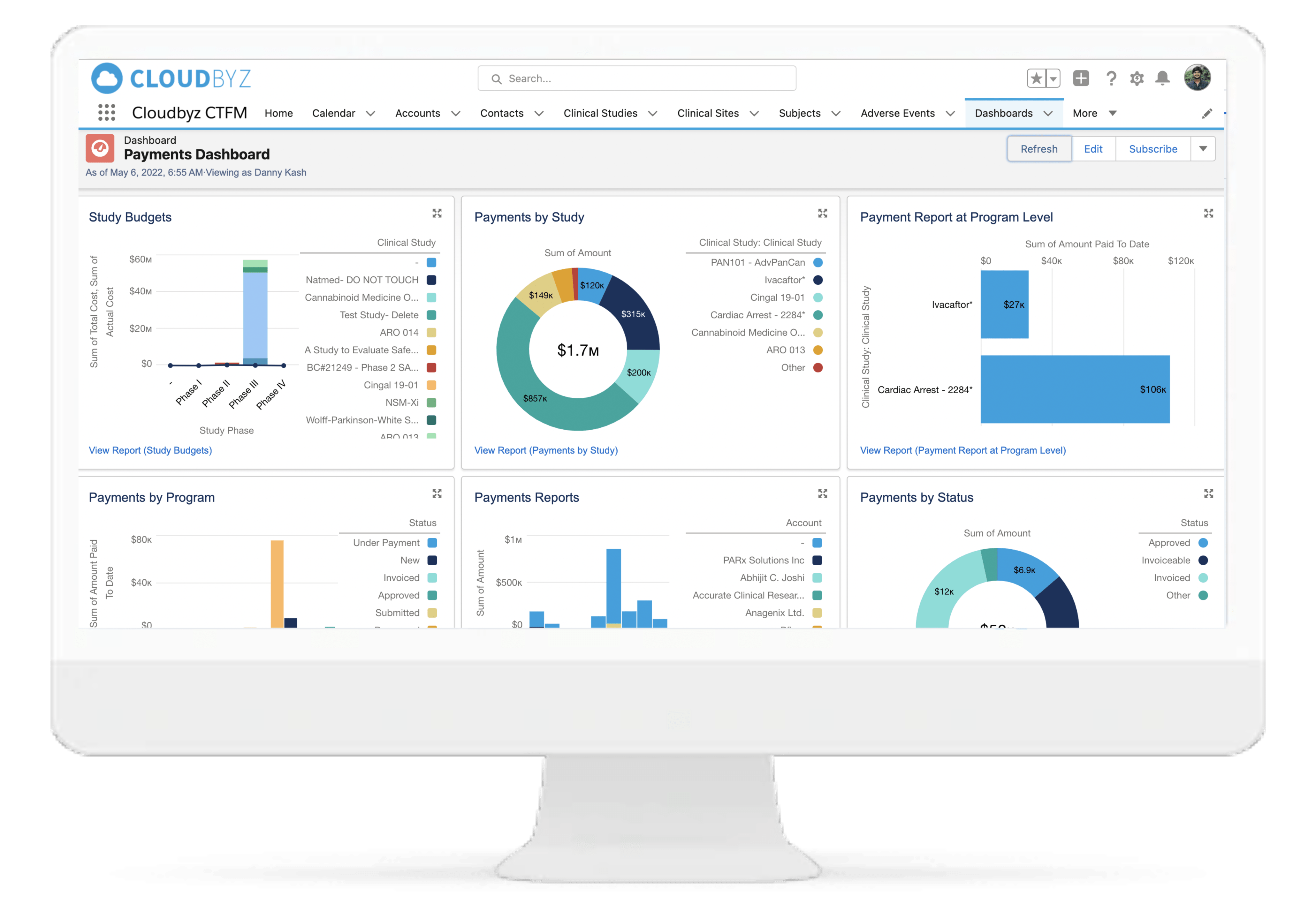
Real-Time Oversight
Centralized dashboards for finance teams, clinical ops, and sponsor stakeholders reduce delays and improve fiscal governance.