Electronic Trial Master File
Animal health clinical trials demand rigorous oversight and audit-ready documentation across diverse study types—from companion animal safety studies to livestock field trials. Cloudbyz eTMF, built natively on Salesforce, offers a unified, cloud-based solution that centralizes document and quality management across the entire study lifecycle. With built-in alignment to animal health regulatory frameworks and VICH GL9-compliant workflows, our platform helps sponsors and CROs accelerate study start-up, ensure data integrity, and remain inspection-ready at all times.
Product Features
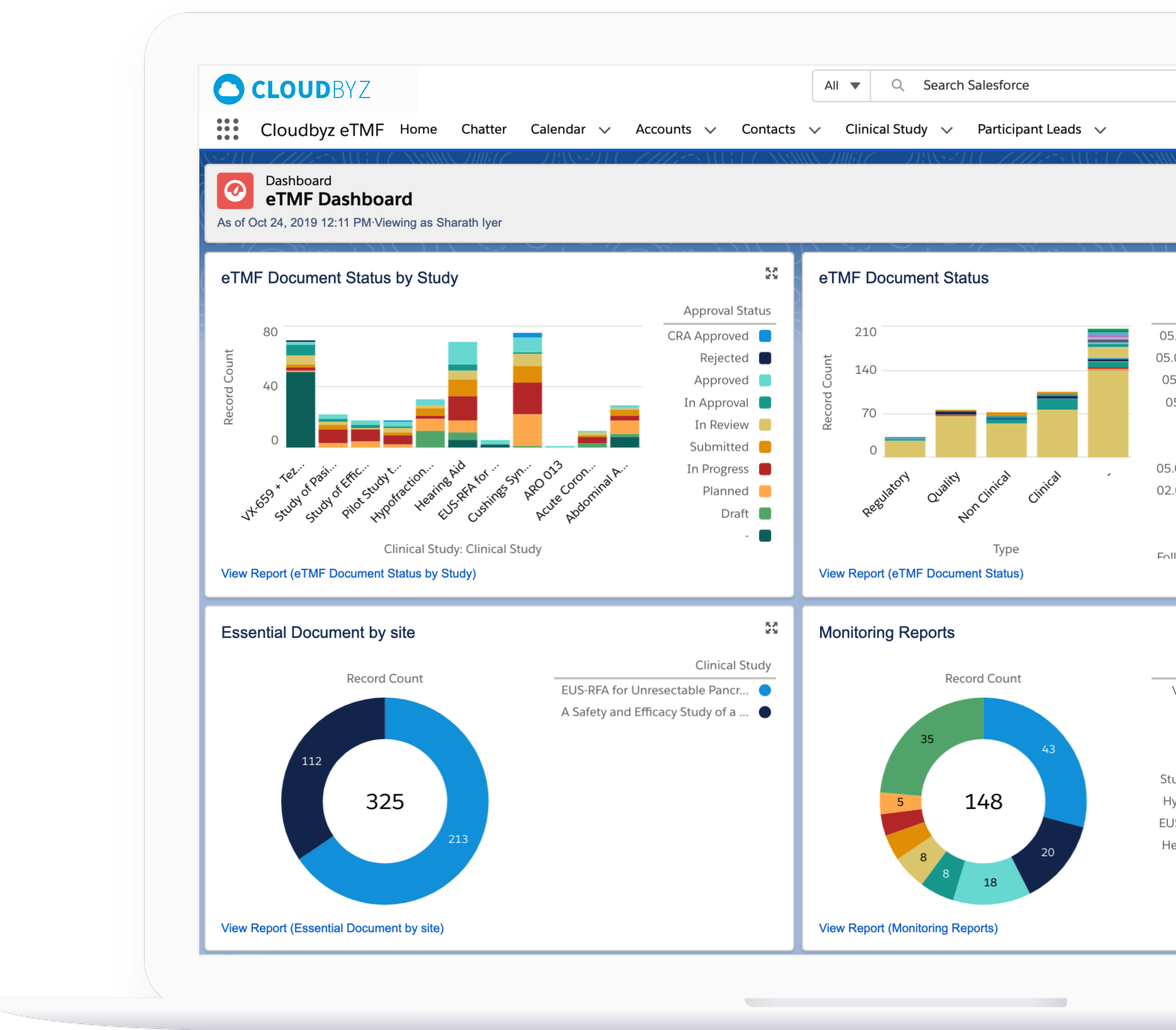
Regulatory Folder Structures for Animal Health Studies
Maintain structured, CVM/EMA-compliant folders specific to TASS, TAES, and multi-species studies. Preconfigured templates align with global animal health requirements and streamline document classification across protocols.
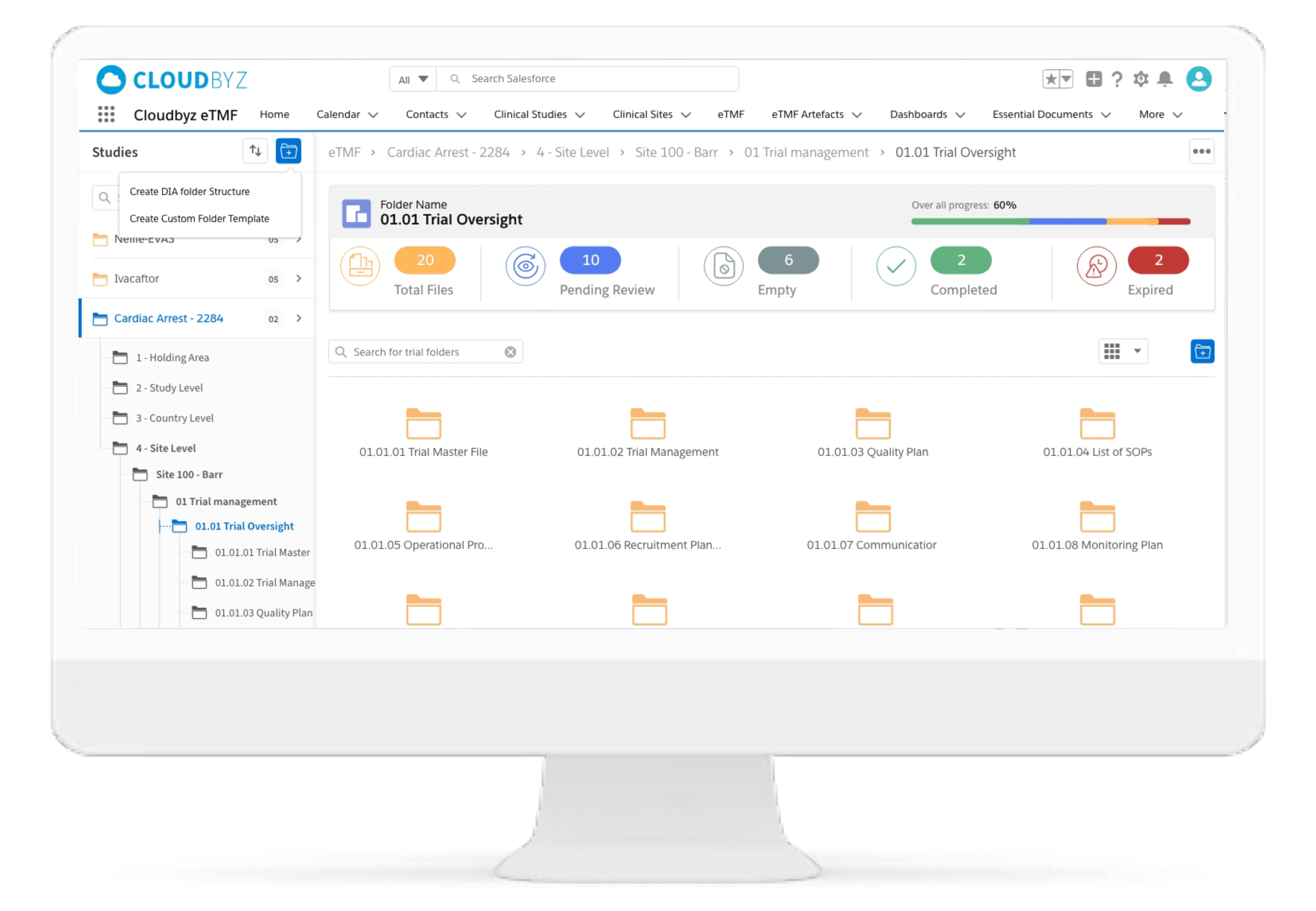
Regulatory Folder Structures for Animal Health Studies
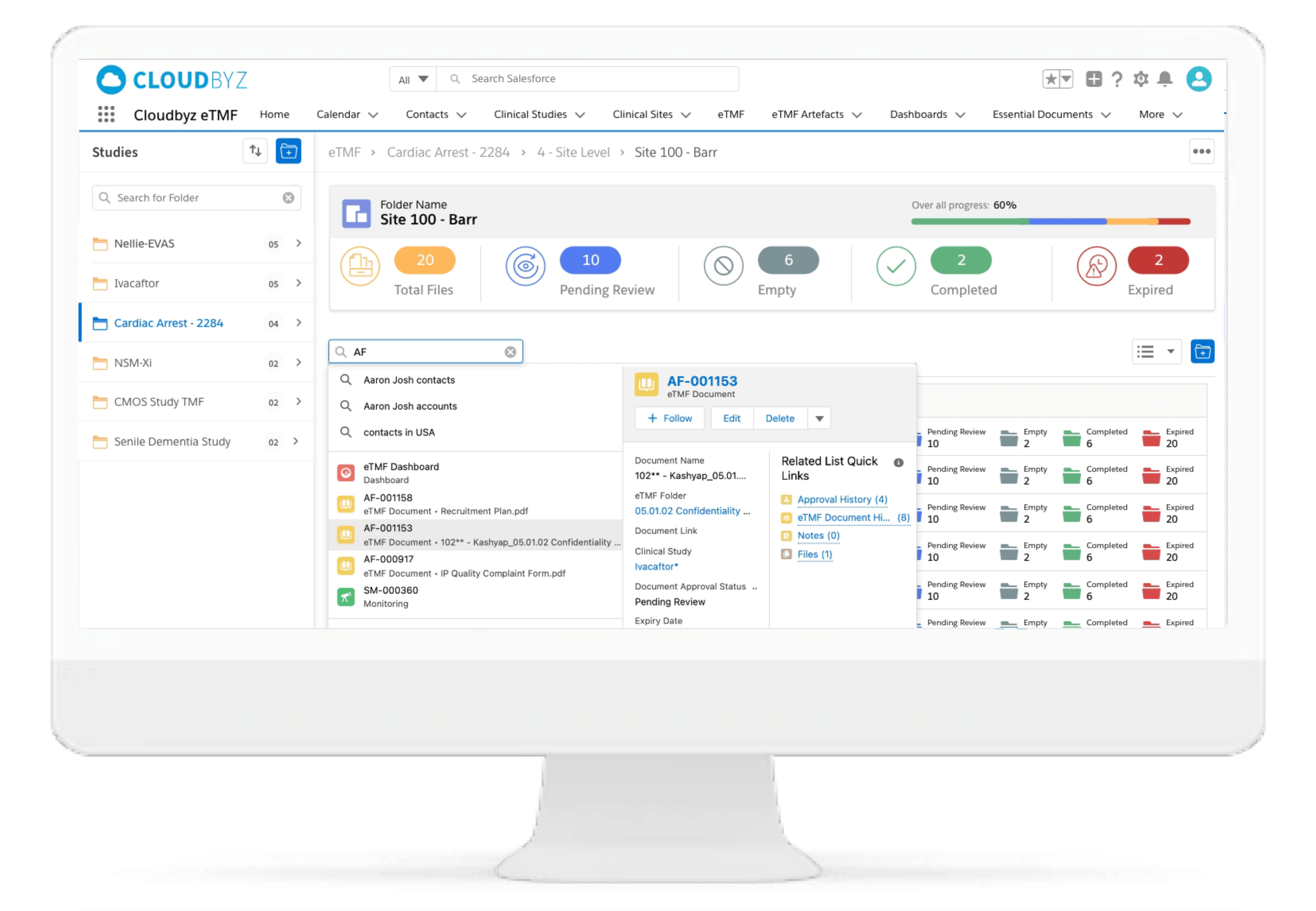
Review & QC Workflow
Built-in workflow routes documents through a review process with a click of a button. Assign document statuses such as "approved", "flagged", "discarded", etc. Send and receive notifications at each step of the review process. Customize the workflow based on the type and nature of a clinical research study.
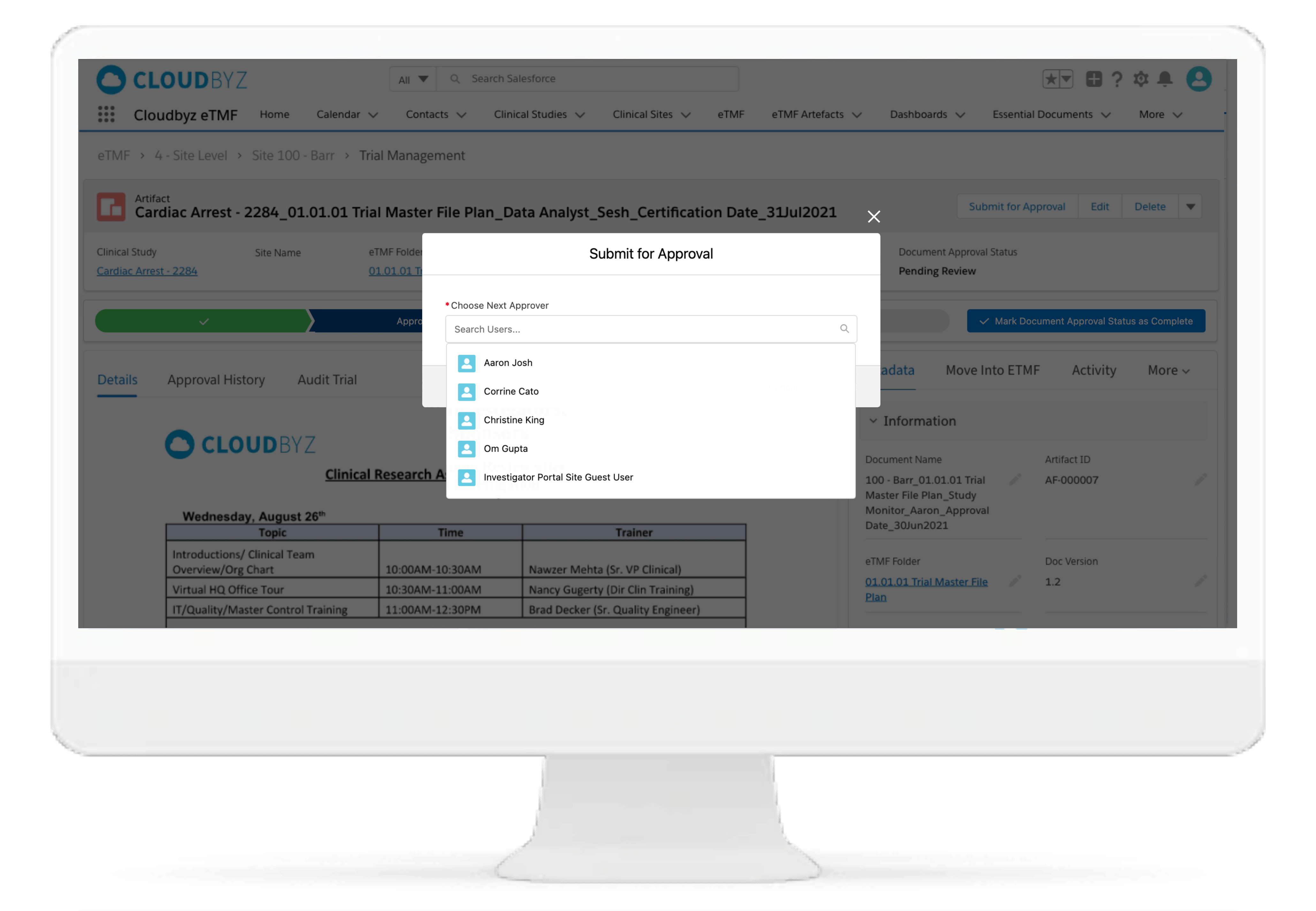
Review & QC Workflow
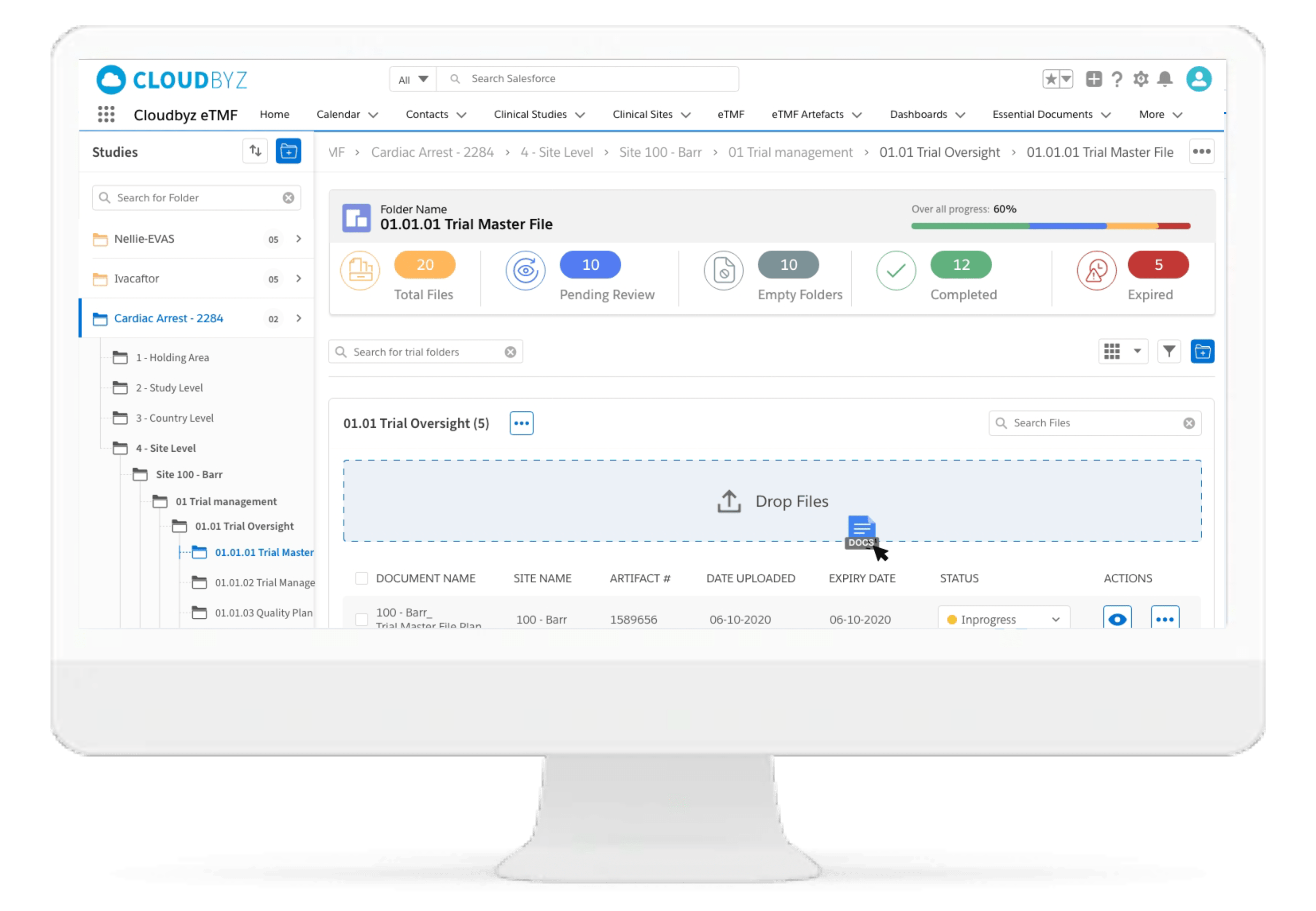
Drag & Drop Upload
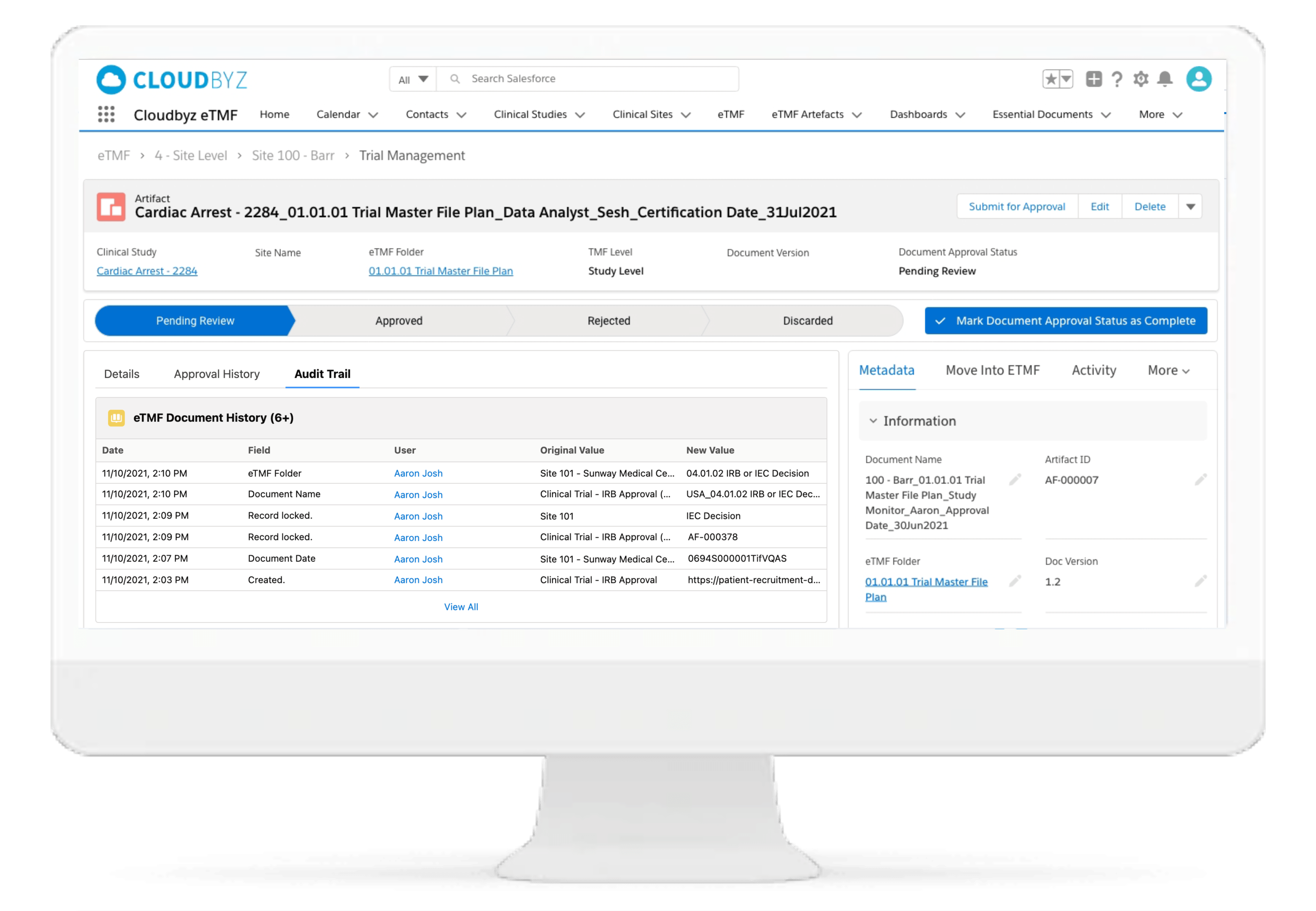
Automated and Configurable Metadata
The solution enables research teams and stakeholders to see content and metadata side by side to eliminate misidentified documents and incorrect metadata. It empowers them to customize, define, and store an unlimited amount of metadata, as well as enabling them to access comprehensive metadata that offers auditors, inspectors, and users, a powerful and dynamic search capability for quick retrieval of documents. Auto-populate a study’s metadata to the maximum extent possible.
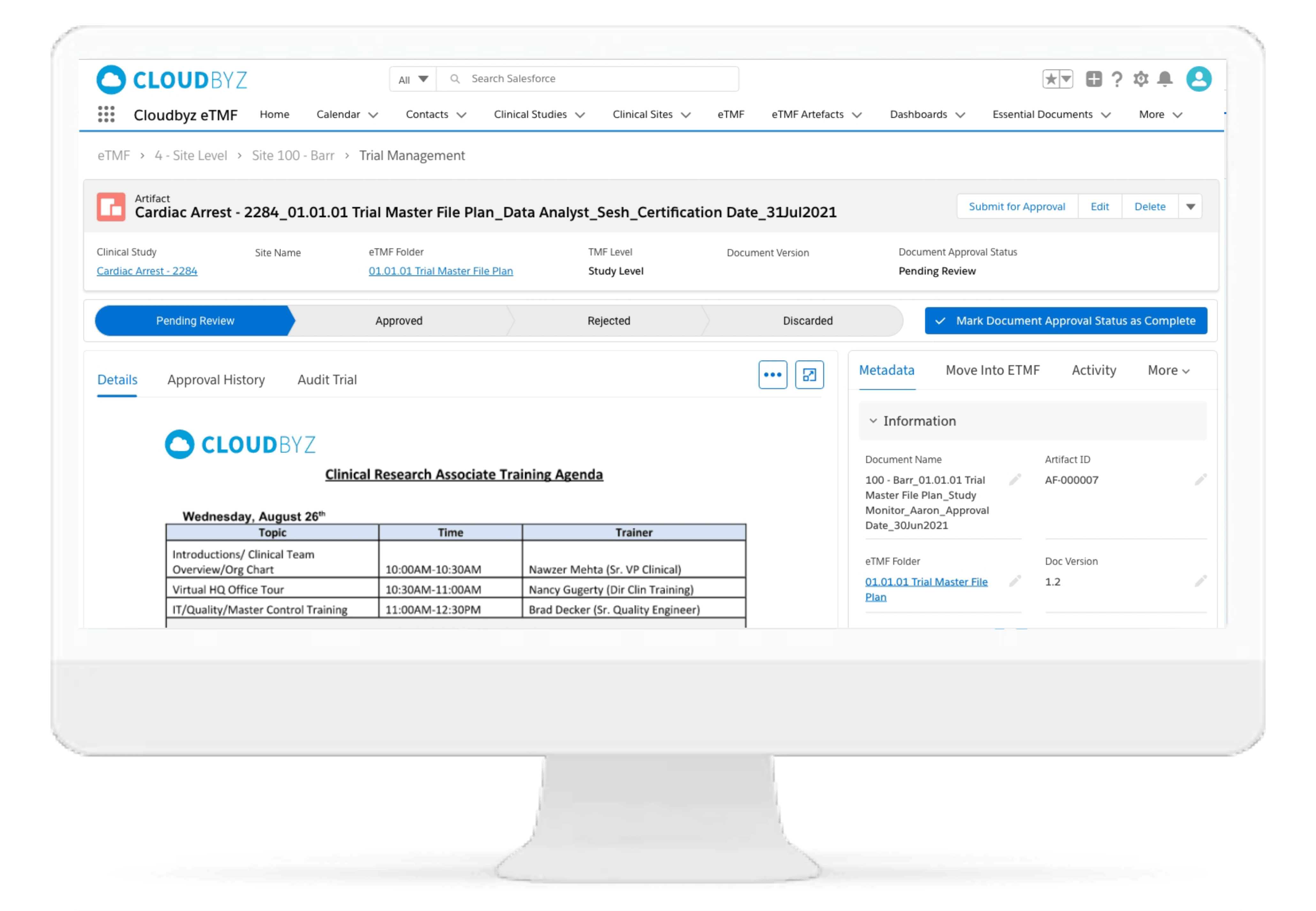
Automated and Configurable Metadata
Integrated Collaboration with CTMS and EDC
Seamlessly link trial master files with data from Cloudbyz CTMS and EDC modules. eTMF automatically captures documentation from monitoring visits, SDV logs, dosing deviations, and lab data entry across multi-site, multi-species trials.
Integrated Collaboration with CTMS and EDC
Why Cloudbyz eTMF for Animal Health?
Aligns with FDA-CVM, USDA-CVB, and EMA-CVMP regulatory frameworks
Built-in GCP support using VICH GL9 guidance
Audit-ready from protocol to post-authorization
Tailored to the complexities of species-specific animal health studies
Seamlessly integrates with your broader eClinical ecosystem