Animal Health clinical trials are essential to bringing innovative therapeutics, biologics, and diagnostics to market for companion, farm, and minor species—while ensuring compliance with regulatory bodies such as the FDA-CVM, USDA-CVB, and EMA-CVMP.
Cloudbyz offers a unified, cloud-native eClinical platform purpose-built for the animal health industry, supporting Target Animal Safety Studies (TASS), Target Animal Effectiveness Studies (TAES), pilot studies, pivotal field trials, and post-authorization surveillance to help sponsors, CROs, and investigators streamline operations, accelerate timelines, and ensure data integrity across species and geographies.
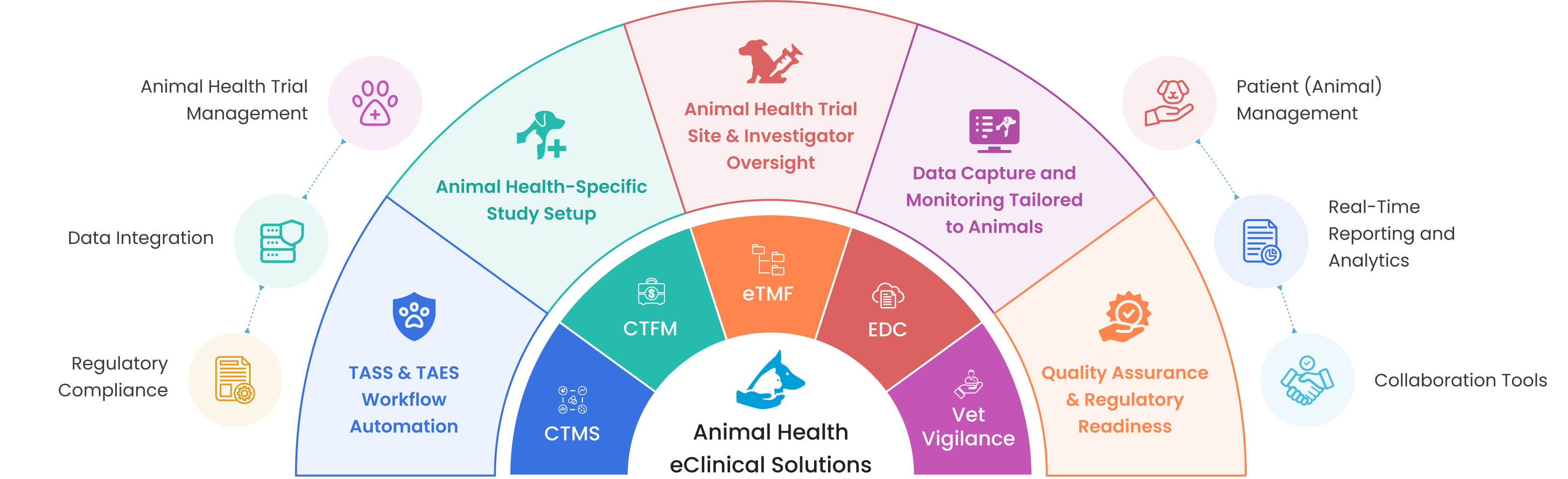
Supporting the Unique Needs of Animal Health Clinical Trials
TASS & TAES Workflow Automation
Manage complete safety and effectiveness study lifecycles with features tailored for animal research:
Dose Escalation Tracking
Enables configurable dosing schedules (e.g., 1x, 3x, 5x) and automated visit templates for monitoring across species. Real-time alerts and validations flag dosing deviations instantly.
Safety Signal Monitoring
Offers AE/SAE tracking using VeDDRA-coded terminology for species-specific accuracy, along with GCP-compliant audit trails.
Efficacy Endpoint Capture
Includes field-ready eCRFs and blinded study arm logic to reduce bias. Integrated diagnostics allow objective data entry like lesion scores and temperature.
Animal Health-Specific Study Setup
Streamline the design and activation of animal studies with tools built for species, sites, and compliance.
Configurable study templates
Configurable study templates tailor pilot and pivotal designs to each species, indication, and regulatory pathway (e.g., TASS, TAES).
Investigator site setup tools
Investigator site setup tools deliver digital feasibility questionnaires, SQV logs, and automated qualification workflows to confirm readiness.
Owner consent tracking
Owner consent tracking captures audit ready electronic signatures, applies inclusion/exclusion rules, and logs deviations in real time.
Animal Health Trial Site & Investigator Oversight
Digital tools to monitor site performance, delegate responsibilities, and ensure protocol adherence in real time.
Digital MVR and SDV workflows enable real-time monitoring
Integrated site monitoring tools support digital Monitoring Visit Reports (MVRs) and Source Data Verification (SDV), helping sponsors and CROs track compliance, enrollment progress, and data accuracy.
Role-based delegation logs and task assignment for animal health site teams
Built-in Delegation of Authority (DOA) logs allow Principal Investigators (PIs) to assign roles and responsibilities to animal health staff, ensuring that each task is tracked and auditable.
Automated alerts for enrollment, deviations, and follow-up
Trigger real-time notifications for key milestones such as subject enrollment, visit scheduling, protocol deviations, or missed assessments.
Data Capture and Monitoring Tailored to Animals
Seamlessly collect, track, and manage species-specific clinical data across diverse study environments.
Multi-Species Patient Records
Capture patient records across multiple species and breeds with structured, species-specific fields. The platform ensures accurate and relevant documentation throughout the study.
Species-Specific Parameter Monitoring
Track vitals, pathology, and behavioral indicators customized to the target species and study goals. Forms are tailored to capture meaningful clinical endpoints.
Field-Ready Mobile Data Capture
Enable real-time data entry for TAES studies conducted in field or clinic settings. Mobile access ensures flexibility for livestock and companion animal trials.
Quality Assurance & Regulatory Readiness
Ensure every study action is compliant, auditable, and aligned with global animal health standards.
Audit-Ready Documentation
Comprehensive logs and real-time reports keep every action traceable, ensuring you’re inspection-ready for CVM, CVB, or EMA reviews.
Role-Based Control & Versioning
Granular access permissions safeguard sensitive study data, while automatic document versioning preserves full revision history.
Global GCP Compliance
Built-in VICH GL9 rule sets guide workflows across all study phases. Configurable checklists and alerts help sites maintain international quality standards with minimal overhead.
Our Solutions
Our unified, Salesforce-powered eClinical platform is purpose-built for managing animal health clinical trials across companion, livestock, and minor species. From pilot to post-approval studies, it centralizes trial operations, data capture, safety tracking, budgeting, and regulatory compliance—empowering sponsors and CROs to streamline execution, enhance oversight, and foster cross-site collaboration.
CTMS
Seamless End-to-End Clinical Trial Operations
Manage animal health-specific workflows including TASS and TAES, automate digital MVRs, SDVs, and protocol compliance, and track dosing, enrollment, deviations, and follow-up across multi-species trials.
CTFM
Real-Time Visibility on Budgets and Payments
Track investigator payments, vendor budgets, and study milestones in real time. Designed for the animal health industry, it helps maintain fiscal oversight across field-based and multi-country animal health trials.
eTMF
Improve Trial Management with Real-Time Collaboration
Ensure inspection readiness with version-controlled documents, digital signatures, and audit trails. Built-in regulatory folders support CVM/ EMA-aligned structures tailored for animal health studies.
EDC
Accelerate Study Build and Data Capture
Capture species-specific clinical data with field-ready eCRFs. Support blinded/randomized study arms, multi-species inputs, and lab integrations for endpoints like lesion scoring, temperature, and pathogen loads.
Vet Vigilance
Unified Safety Adjudication and Reporting
Track AEs and SAEs using VeDDRA-coded terminology across species. Log safety parameters like behavior or organ function, and maintain GCP-compliant audit trails to support regulatory reporting for animal health products.
Key aspects of our solution include
Animal Health Trial Management
Streamline every phase of animal health studies—from protocol setup and site management to multi-species trial execution and reporting—on one unified platform.
Data Integration
Connect seamlessly with animal health EMRs, lab systems, and diagnostic tools to centralize multi-source data and enhance accuracy across species and trial sites.
Regulatory Compliance
Maintain full alignment with FDA-CVM, USDA-CVB, EMA-CVMP, and VICH GL9 standards to ensure regulatory-grade data capture and inspection readiness.
Patient (Animal) Management
Track health status, treatment progress, and behavioral or clinical observations of animal subjects with species-specific fields and scheduling tools.
Real-Time Reporting and Analytics
Leverage built-in dashboards and analytics to monitor enrollment, adverse events, and endpoint outcomes for timely insights and faster decisions.
Collaboration Tools
Enable seamless coordination across sponsors, CROs, veterinarians, and field investigators through role-based access, alerts, and centralized documentation.
Benefits
Operational Efficiency
Digitize and streamline animal health trial workflows, reduce administrative burden, and optimize resource use across species and study types.
Animal Welfare Assurance
Ensure the highest standards of care with built-in monitoring, species-specific tracking, and welfare-centric workflows for all enrolled animals.
Reliable Data Integrity
Maintain consistency and traceability of clinical data across multiple sites and species, enabling faster and more confident decision-making.
Regulatory Confidence
Achieve full compliance with FDA-CVM, USDA-CVB, and EMA-CVMP regulations, minimizing audit risk and enhancing trial credibility.
Connected Research Ecosystem
Strengthen collaboration between sponsors, CROs, and veterinary sites with integrated tools for real-time communication and task management.
Accelerated Scientific Innovation
Harness structured, real-time data to fuel new insights in veterinary medicine and advance therapeutic innovation in animal health.

