Clinical Trial Management System
Animal health studies—from Target Animal Safety Studies (TASS) and Target Animal Effectiveness Studies (TAES) to pilot and pivotal field trials—require operational agility, regulatory compliance, and species-specific workflows. Cloudbyz CTMS offers a unified, cloud-native platform designed specifically to manage the complexities of animal health clinical research.
Product Features
Study Planning & Design
Create animal health specific study plans with prebuilt templates tailored for pilot, pivotal, TASS, and TAES studies. Configure workflows to reflect species, indication, and regulatory pathway—ensuring alignment with CVM, CVB, or EMA-CVMP requirements.
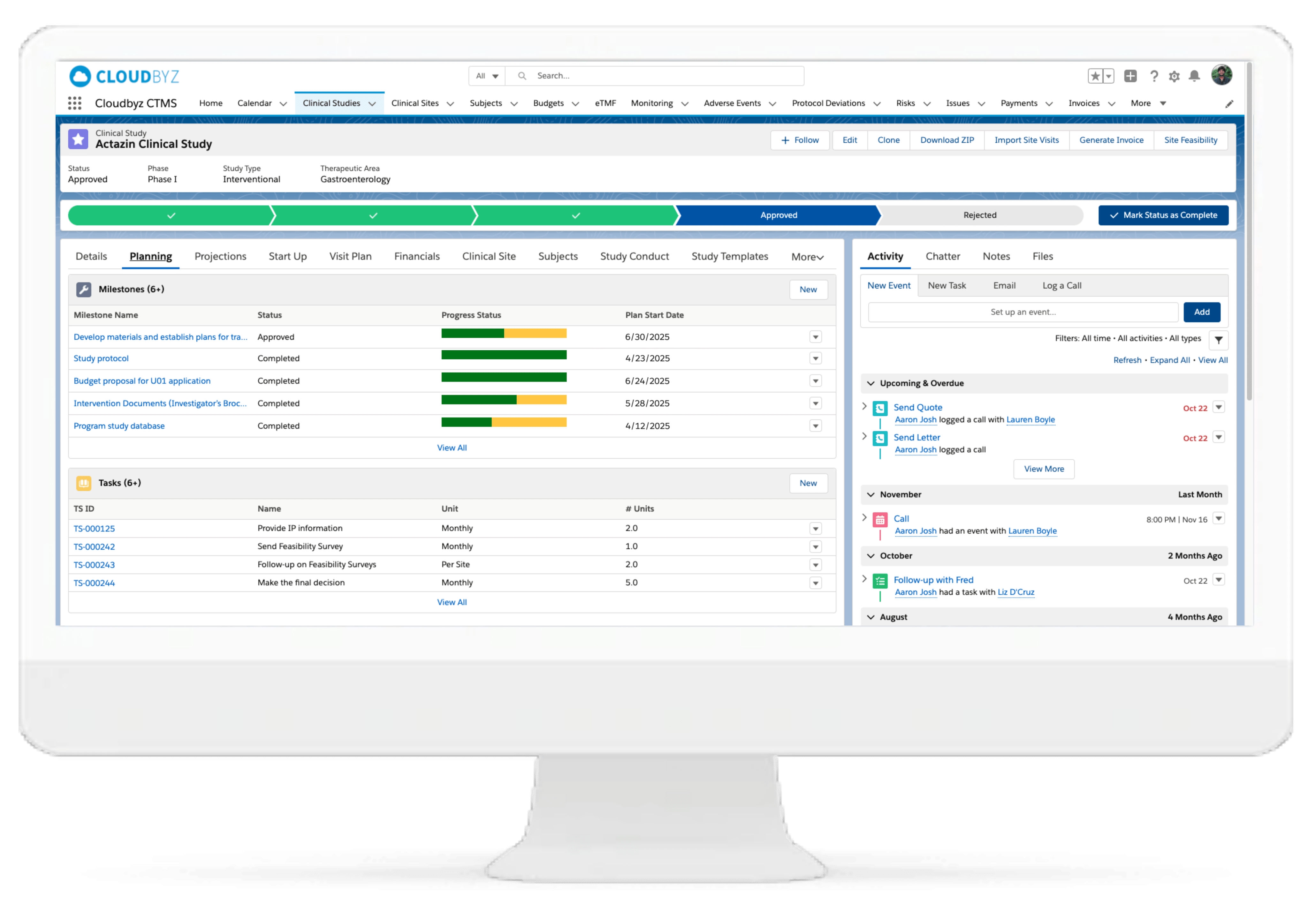
Study Planning & Design
Study Planning & Design
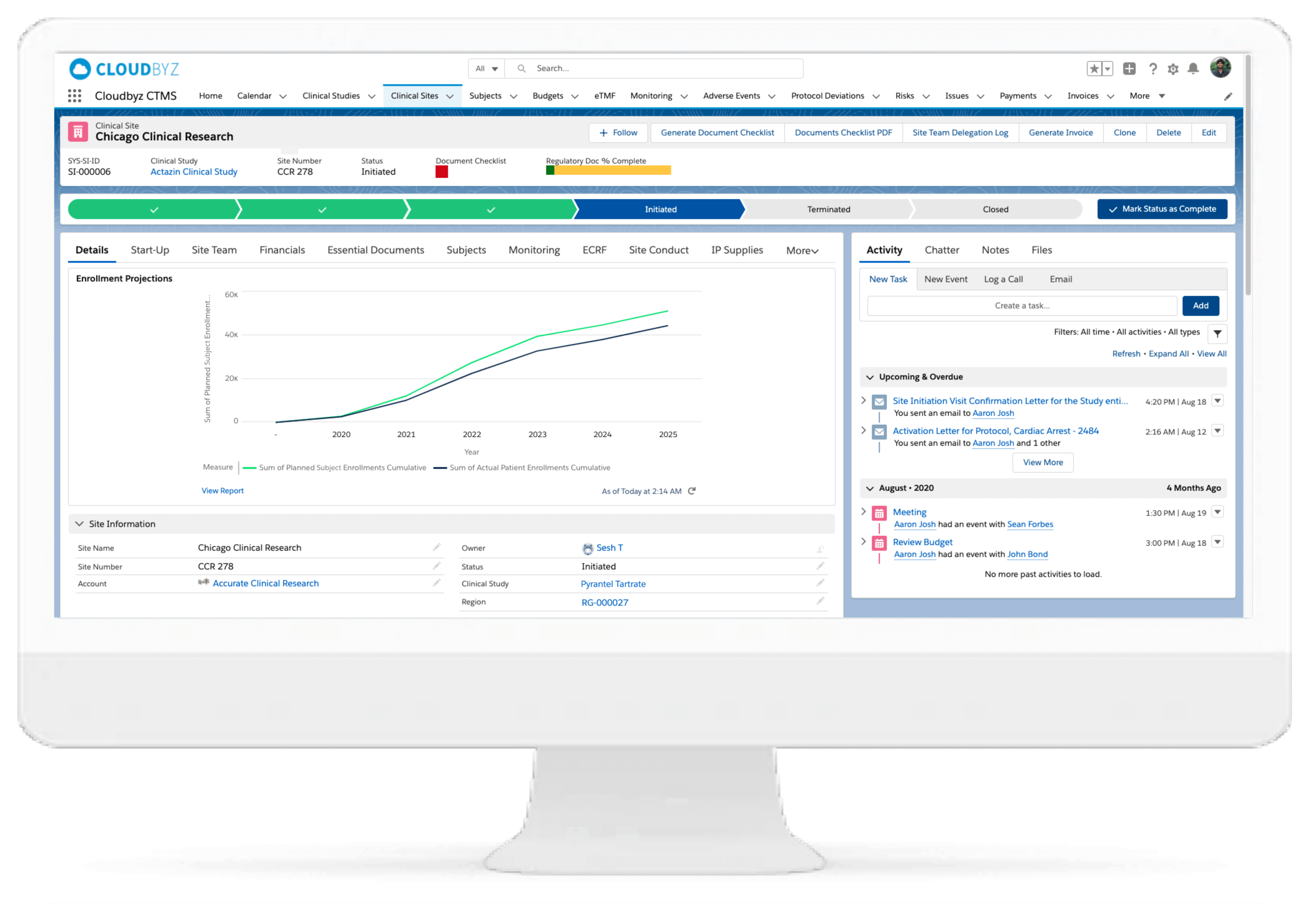
Multi-Species Site Management
Multi-Species Site Management
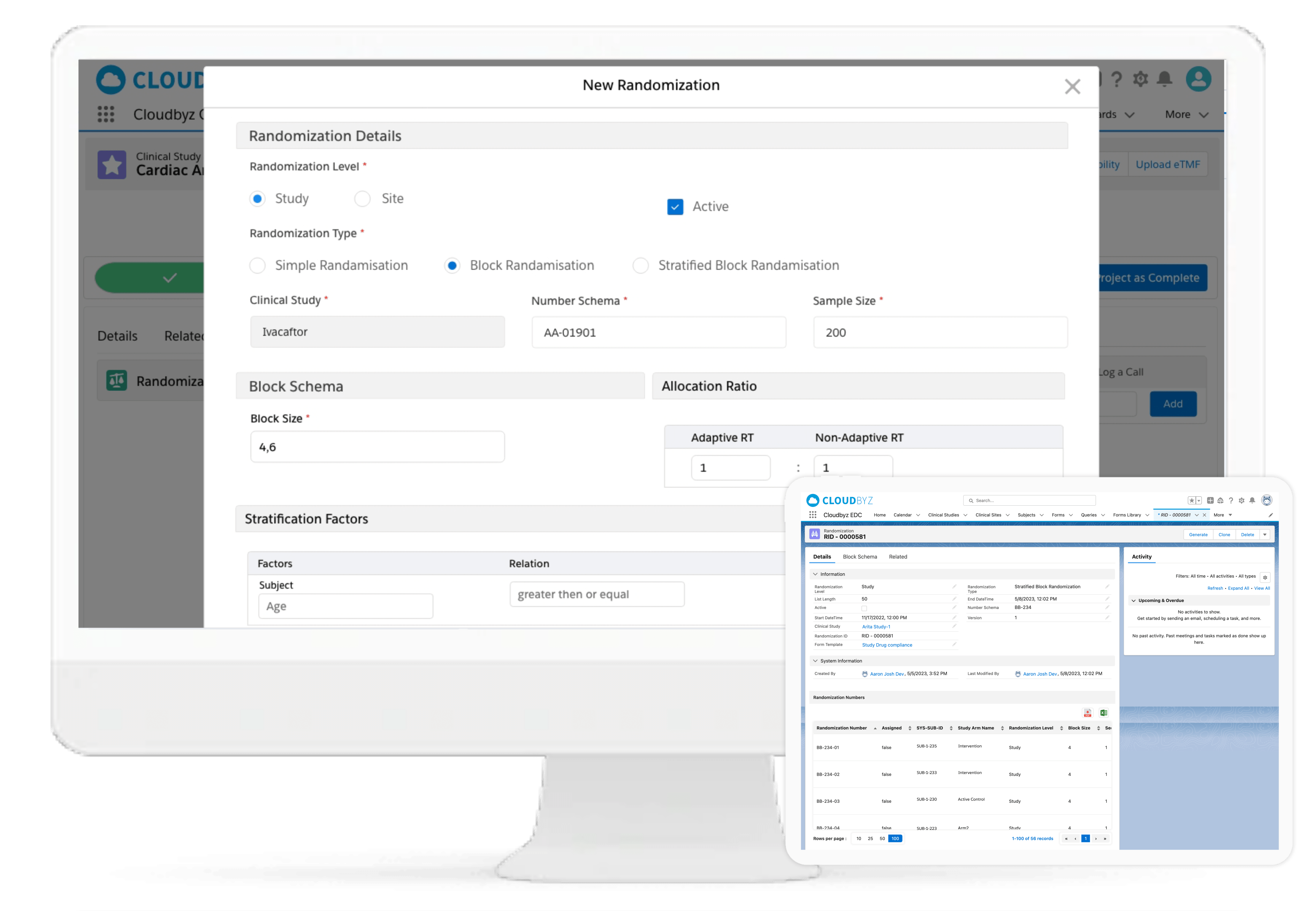
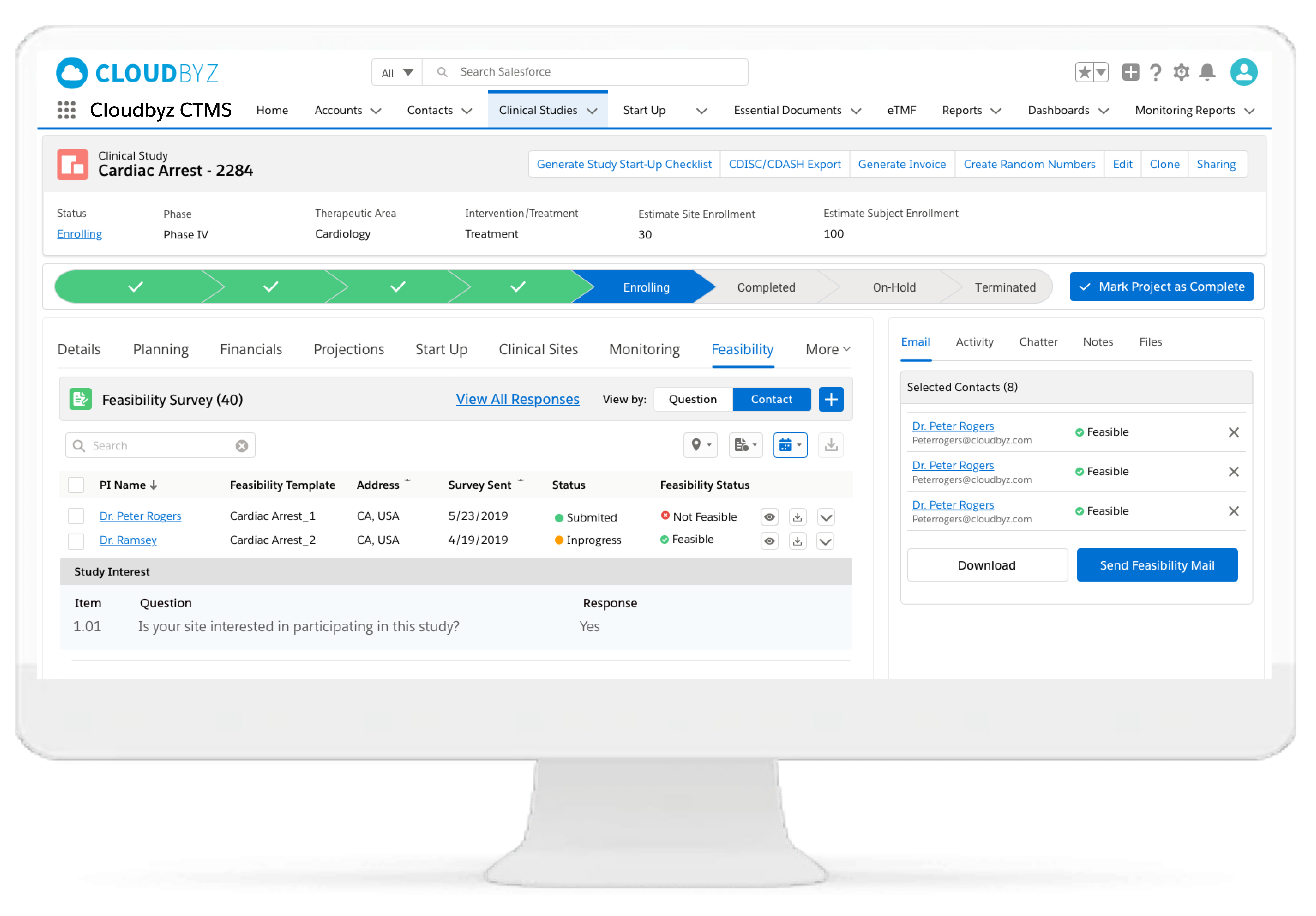
Protocol & Visit Management
Define and track multi-species visit schedules, treatment windows, and protocol-specific activities. Configure dosing cohorts (e.g., 1x, 3x, 5x) for target species and receive real-time alerts for dosing variances or visit delays.
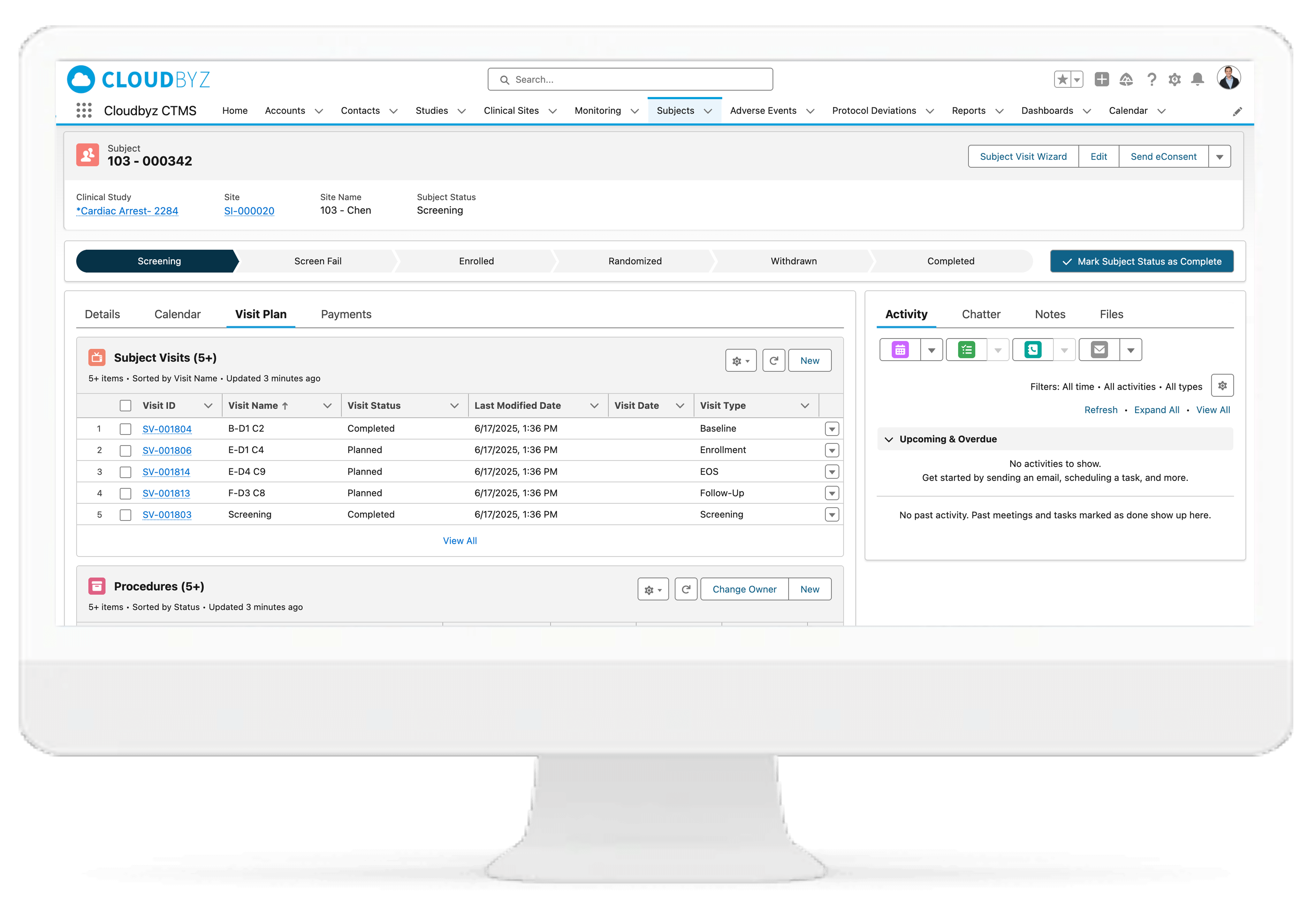
Protocol & Visit Management
Subject (Animal) Lifecycle Tracking
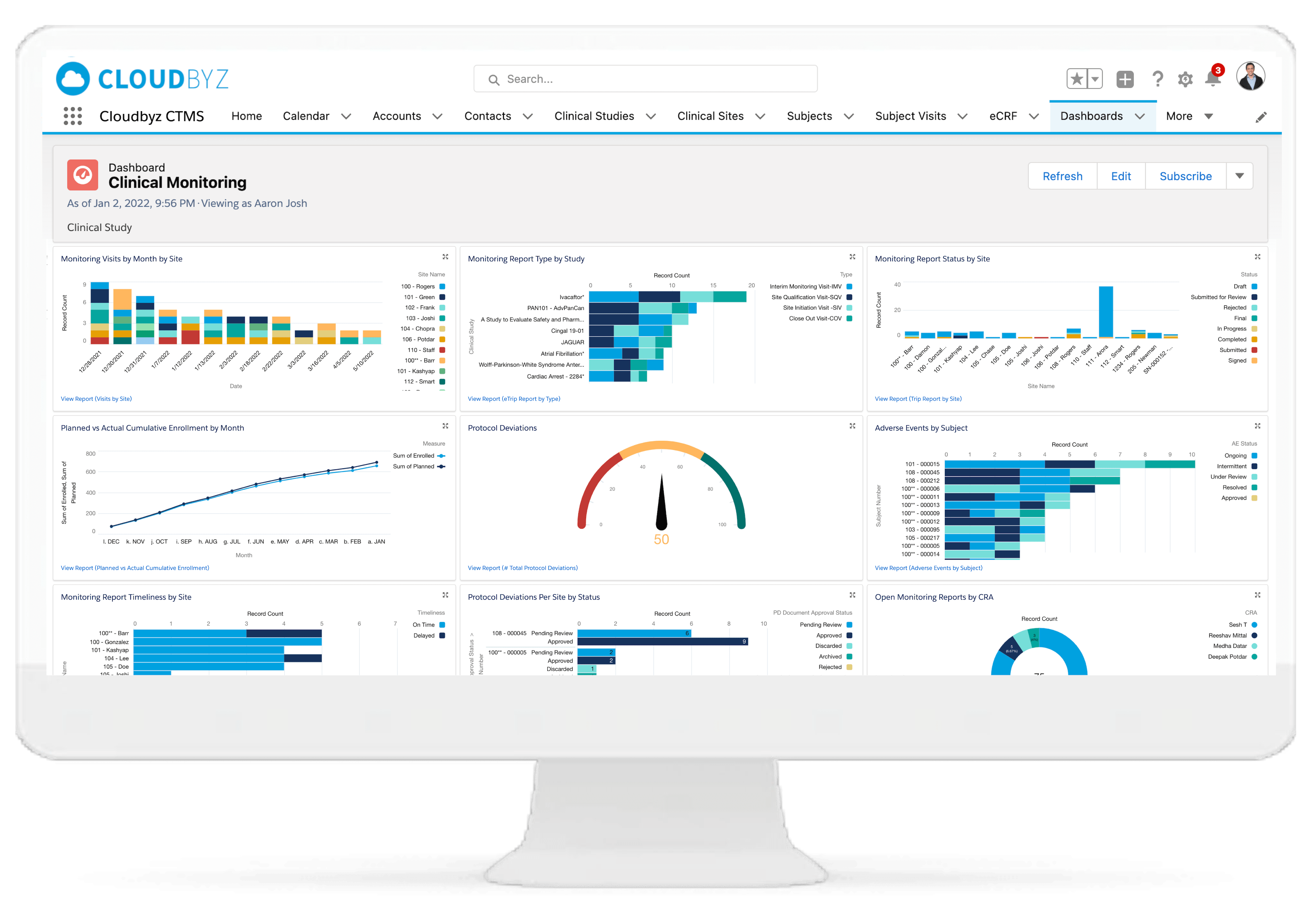
Serious Adverse Event (SAE) Tracking
Log AEs/SAEs using VeDDRA-coded species terminology. Capture investigator notes, attach diagnostics, and track resolution timelines. All activity is logged with GCP-compliant audit trails for regulatory reporting.
Serious Adverse Event (SAE) Tracking
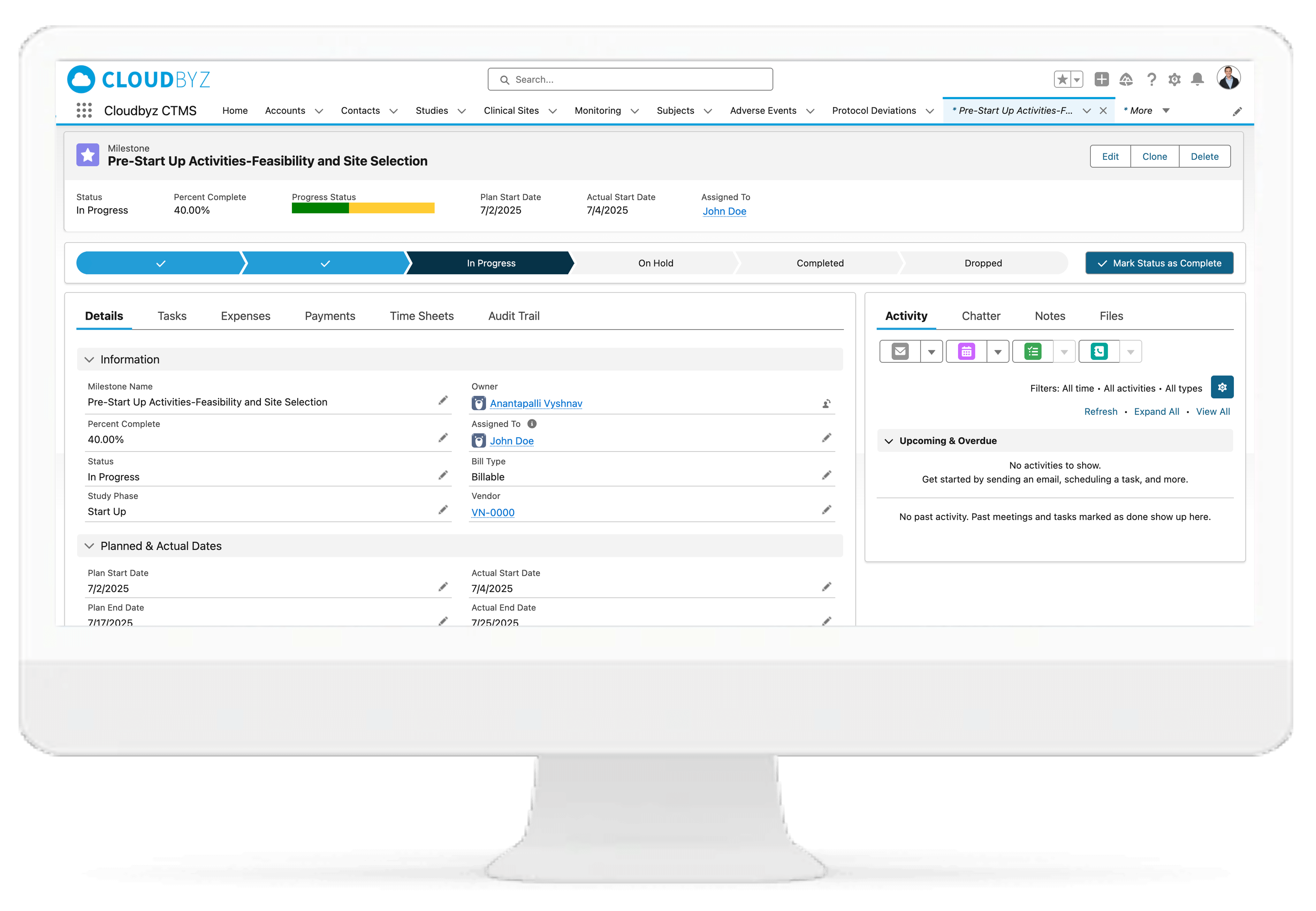
Milestone & Activity Management
Audit Trails & Inspection Readiness
Maintain a fully auditable record of all trial actions, from owner consent to protocol deviations. Version-controlled documents, time-stamped actions, and regulatory folders prepare you for CVM, CVB, or EMA inspections.
Audit Trails & Inspection Readiness
Why Cloudbyz CTMS for Animal Health?
Built for Multi-Species Trials
Configurable to support dogs, cats, cattle, poultry, and other companion and farm animals
Fully Compliant
Designed to align with FDA-CVM, USDA-CVB, EMA-CVMP, and VICH GL9 regulations
Unified Oversight
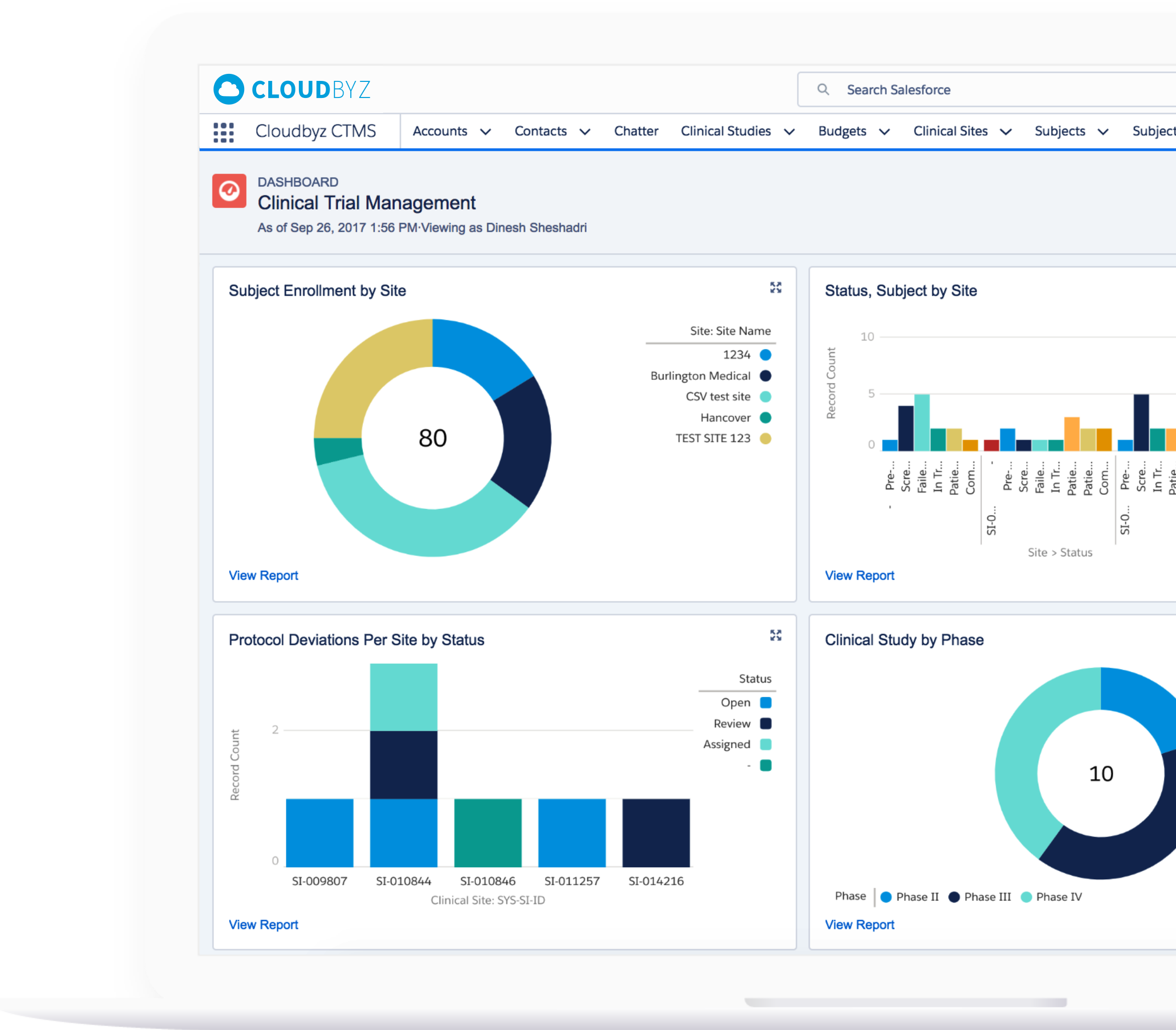
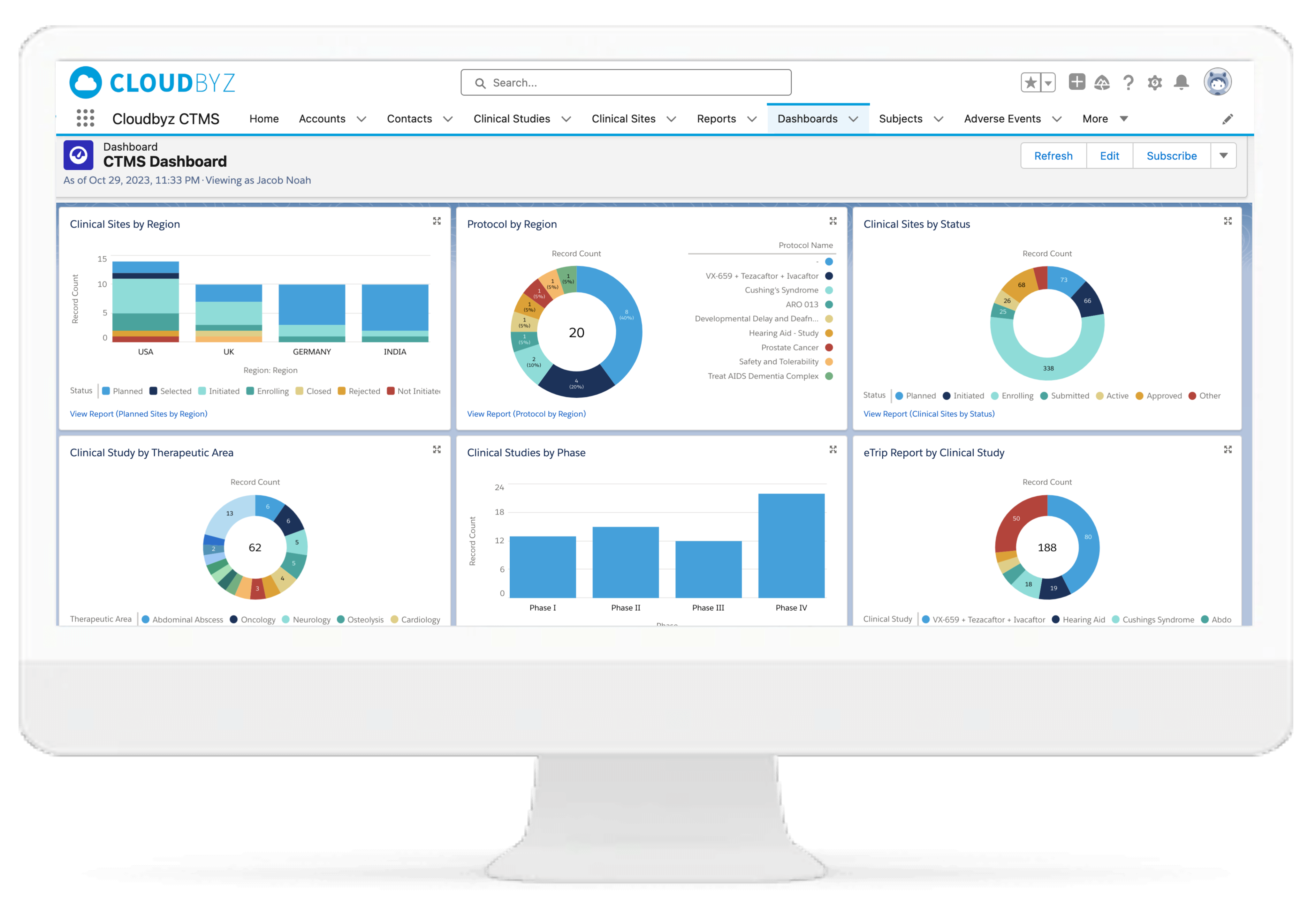
Monitor study progress, protocol compliance, safety, and data quality from a centralized dashboard
Mobile-Enabled
Support in-field data capture for TAES studies with or without network connectivity
Secure & Scalable
Salesforce-native platform ensures data integrity, scalability, and rapid deployment across global sites