Cloudbyz Veterinary Vigilance Solution
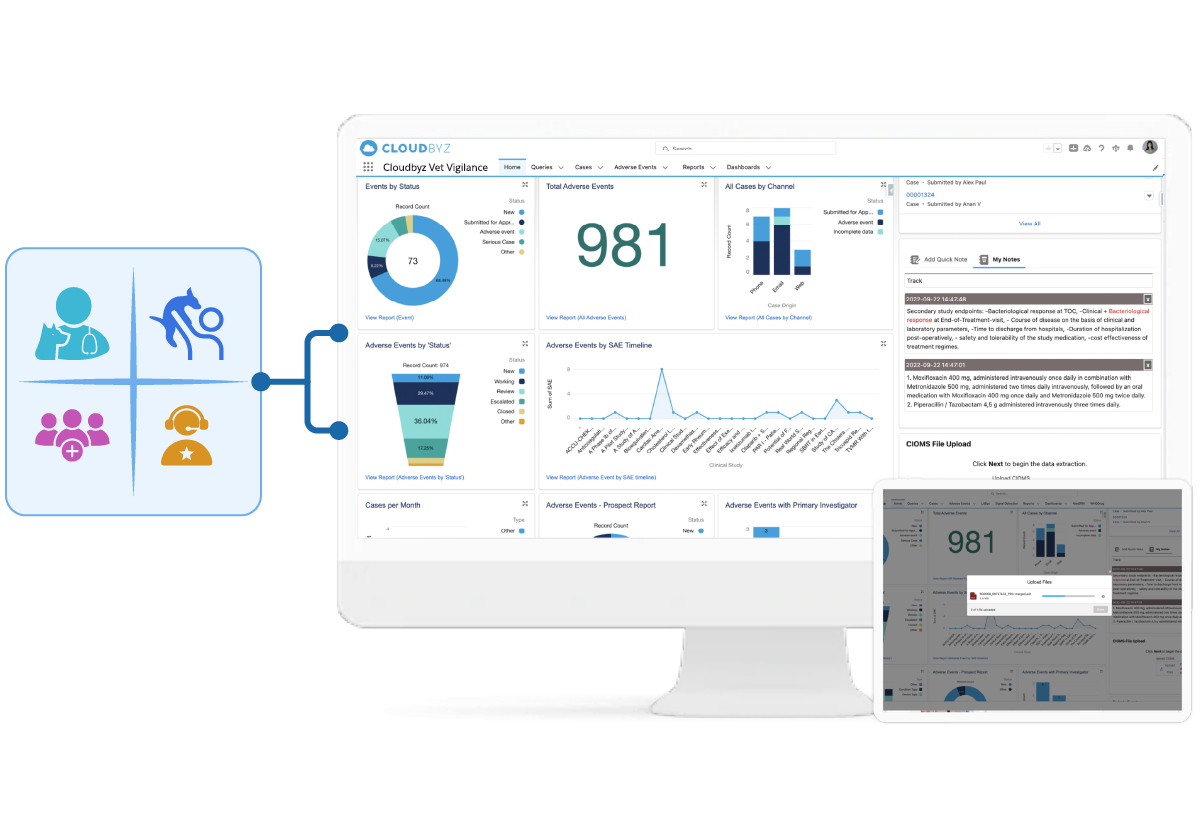
The Cloudbyz Animal (Veterinary) Health Pharmacovigilance solution is a cloud-native platform purpose-built for veterinary medicine safety surveillance. Designed for life sciences companies, marketing authorization holders (MAHs), service providers, CROs, and regulatory teams, the platform enables real-time adverse event (AE) collection, processing, and global reporting across companion, livestock, and specialty species.
Whether you're responding to a new safety signal, preparing a PSUR, or reconciling owner-reported cases from distributed geographies, Cloudbyz enables streamlined workflows, compliance with VICH GL24/GL29 and global veterinary regulations (CVM, CVMP, CVB), and cross-functional collaboration from intake to submission.
Product Features
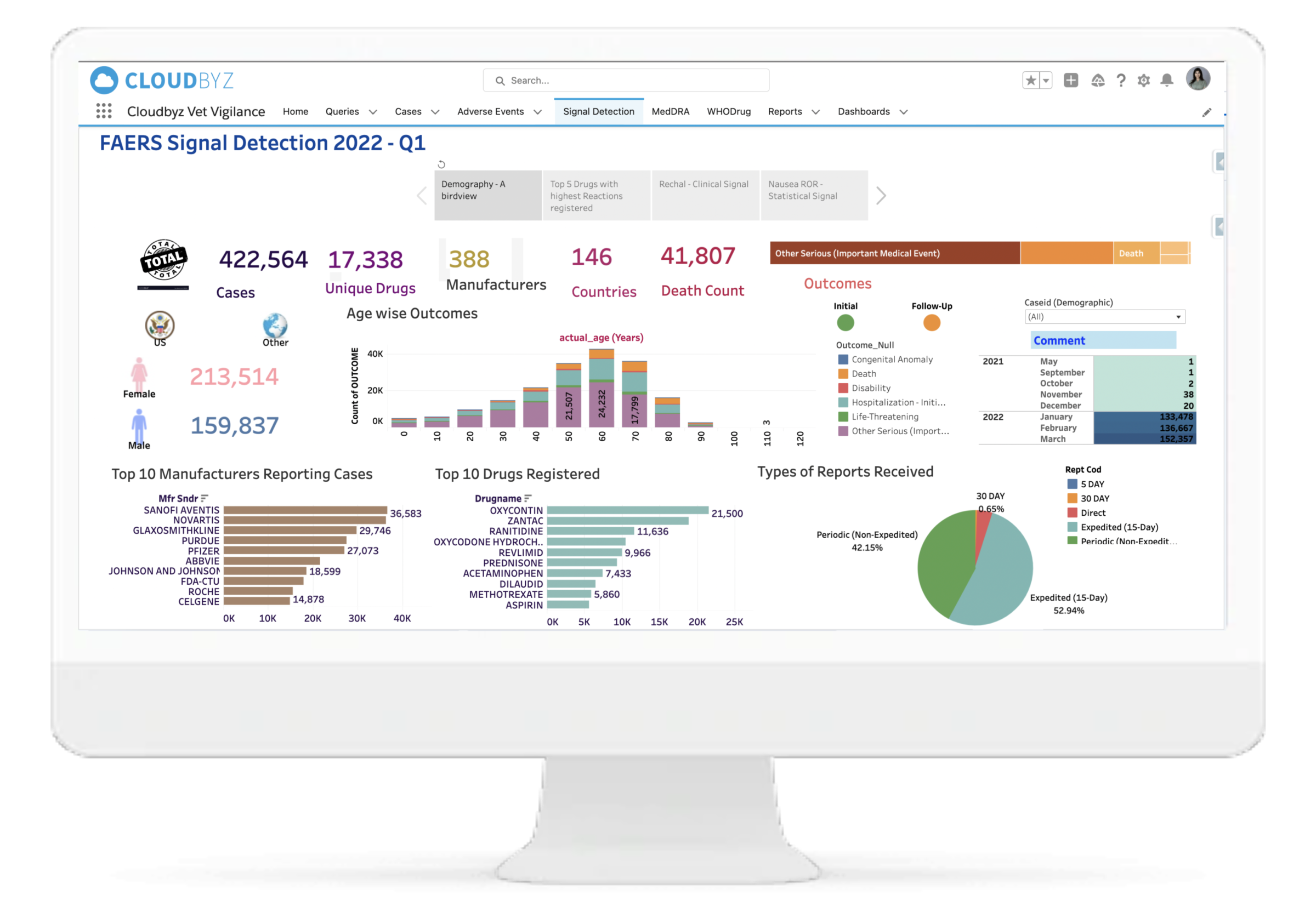
Advanced Querying & Case Search
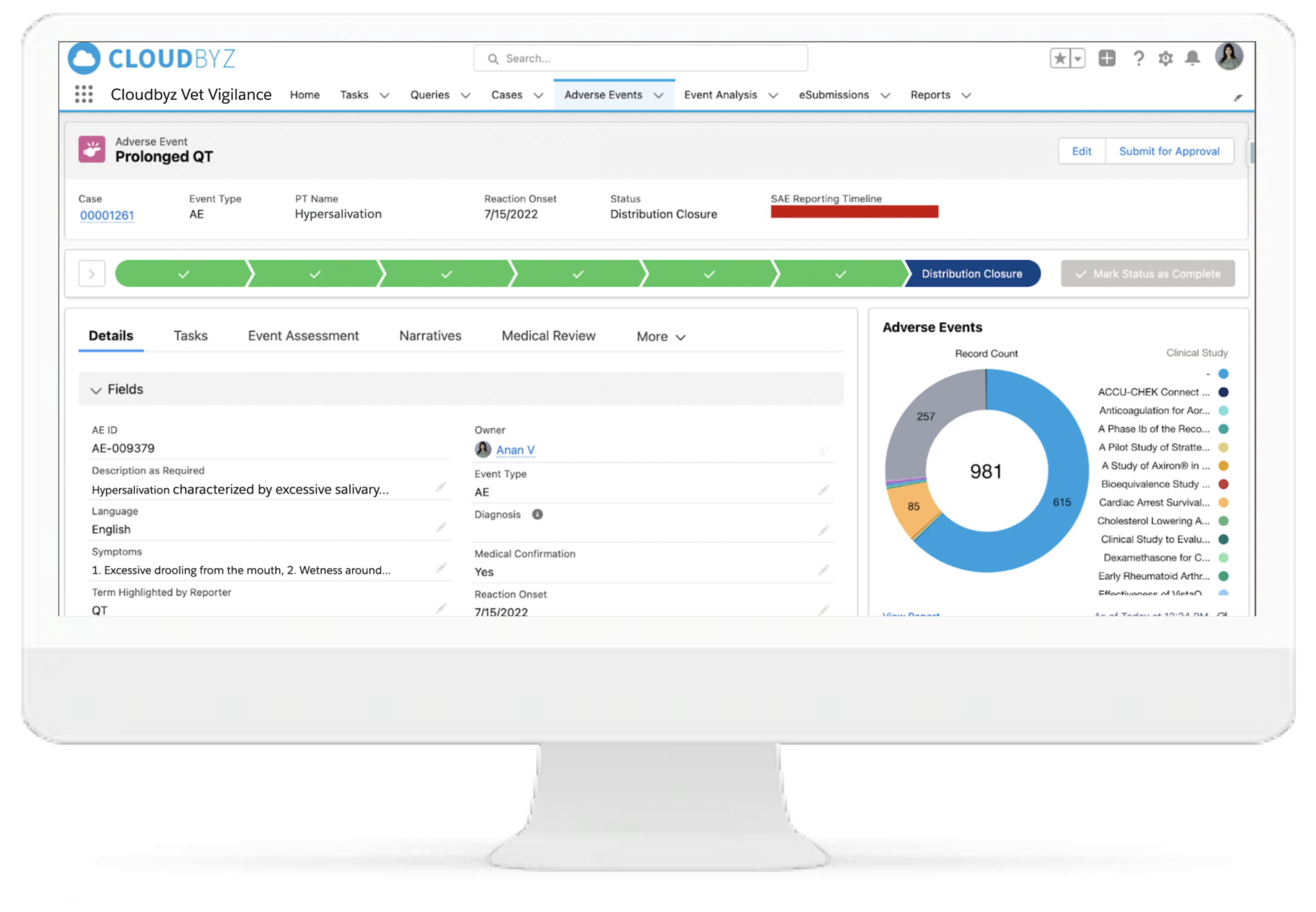
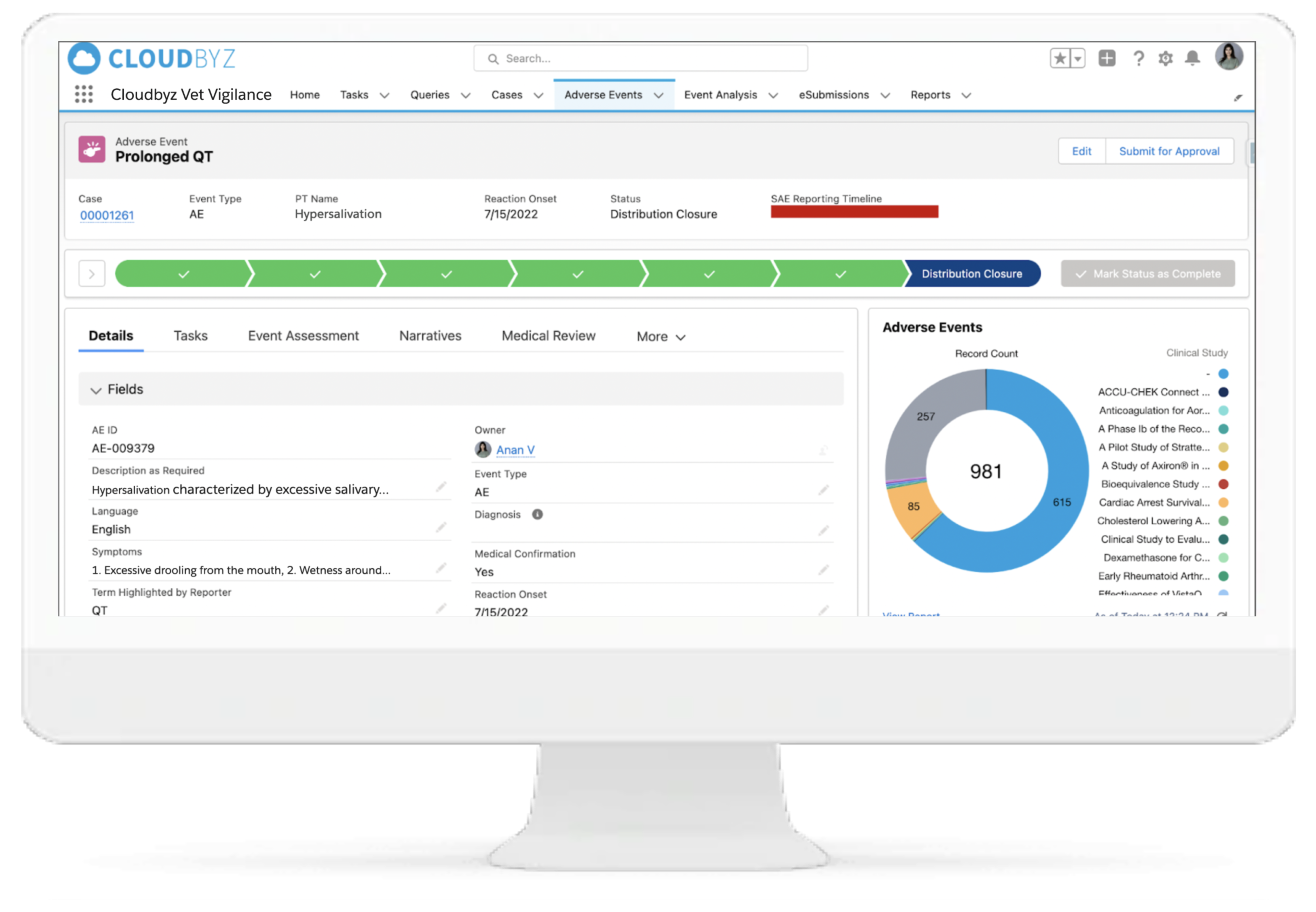
Regulatory-Ready Submissions
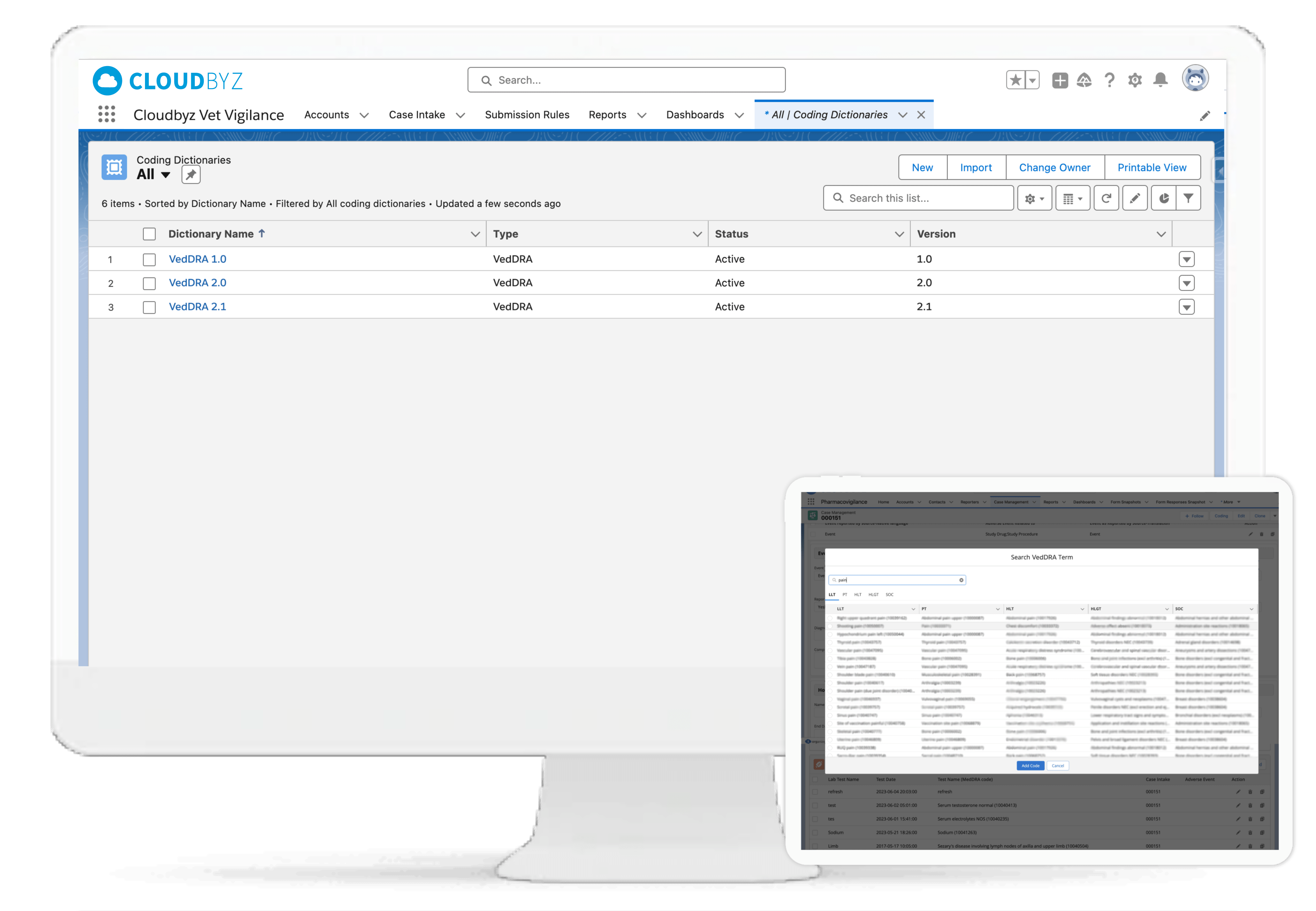
Generate compliant safety reports for FDA CVM, EMA, or USDA CVB. Supports HL7-compliant XML formats, VICH-defined schemas, EU Vet XML, and batch AS2-based transmission. Import and reconcile inbound XML cases from Competent Authorities with full traceability.
Regulatory-Ready Submissions
Key Benefits
Fully aligned with VICH GL24, GL29, CVM, CVMP, and USDA requirements
HL7 and XML-based regulatory submission support
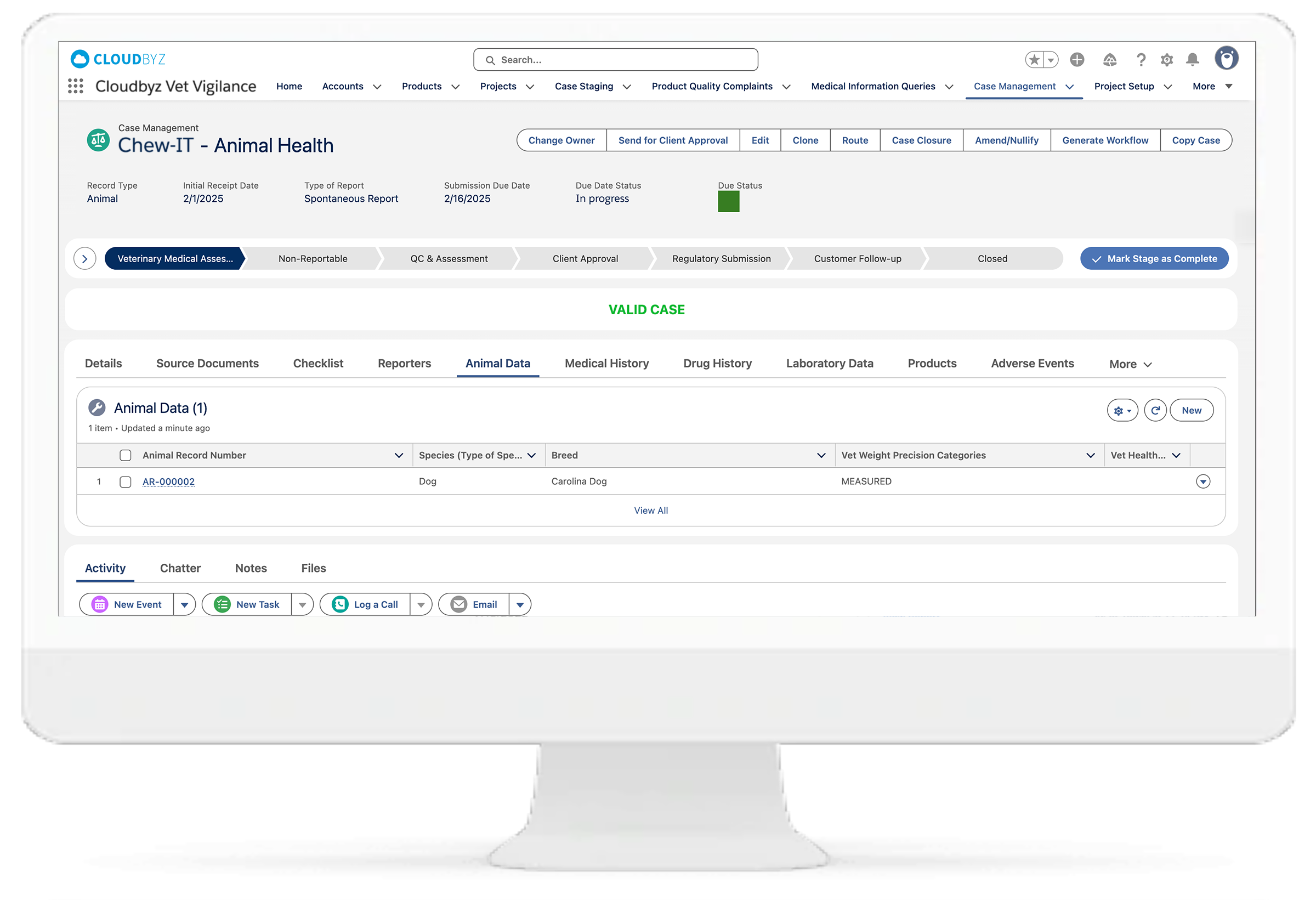
Support for spontaneous, clinical, and post-market cases across species
Built-in signal detection and trend analysis
Unified data environment with integration to Cloudbyz CTMS, EDC, RTSM
Mobile-ready and field-friendly with minimal training needs
.png)